These pellets also contain agents to reduce the potential for ignition or explosion of the released phosphine. Eight 27 of the 30 tunnel workers exposed to gas station vapors developed the disease.
It is also used as a fumigant a polymerization initiator and as an intermediate for the preparation of several flame retardants.
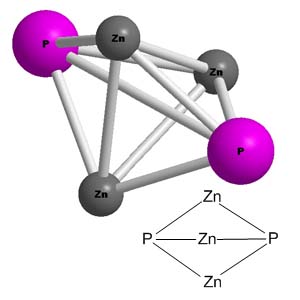
Zinc phosphide phosphine gas. Zinc phosphide Zn 3 P 2 is an inorganic chemical compound. It is a grey solid although commercial samples are often dark or even black. It is used as a rodenticide.
Zn 3 P 2 is a II-V semiconductor with a direct band gap of 15 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system zinc diphosphide ZnP 2. For farm use pellets of aluminium phosphide calcium phosphide or zinc phosphide release phosphine upon contact with atmospheric water or rodents stomach acid.
These pellets also contain agents to reduce the potential for ignition or explosion of the released phosphine. A more recent alternative is the use of phosphine gas itself which requires dilution with either CO 2 or N 2 or even air. Phosphine is used in the semiconductor industry to introduce phosphorus into silicon crystals.
It is also used as a fumigant a polymerization initiator and as an intermediate for the preparation of several flame retardants. Phosphine has an odor of garlic or decaying. Zinc phosphide was first registered in 1947.
1 It changes into phosphine gas in the presence of water and acid. The phosphine gas is very toxic. It blocks the bodys cells from making energy and the cells die.
15 Phosphine exposure is particularly damaging to the heart brain kidney and liver. Phosphine gas exposures to workers may occur when treating. Animals that have ingested rodenticides containing zinc phosphide.
Animals that have ingested insecticides containing aluminum phosphide. AVMA Phosphine product precautions external icon. CDC Morbidity and Mortality Weekly Report MMWR.
Occupational Phosphine Gas Poisoning at Veterinary Hospitals from Dogs. The compound is highly unstable in an acidic environment which ensures a quick disintegration into phosphine gas which results in pulmonary edema vomiting and death. The higher the acidity levels in the stomach the faster the poison will work to exterminate the voles.
If the stomach is empty the zinc phosphide will be absorbed systemically leading to liver and renal failure. In this case. This type of rodenticide is usually used to kill gophers and moles.
When ingested the zinc phosphide is converted to phosphine gas in the stomach. This gas is very irritating to the GI tract so vomiting is usually the first sign. This can be followed by nervous system signs such as ataxia stumbling and balance issues tremors and seizures.
The intact zinc phosphide that is. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. Phosphine gas Potassium n-methyldithiocarbamate metam-potassium labeled for the production of agricultural plant commodities Propanil 34-dichloropropionanilide Sodium cyanide Sodium fluoroacetate compound 1080 unregistered Sodium tetrathiocarbonate unregistered Strychnine Sulfotepp unregistered Sulfuryl fluoride Thiobencarb Tribufos Tributyltin organotin or a tri.
On this page you will be able to access individual Acute Exposure Guideline Level AEGLs values that are intended to protect most individuals in the general population including those that might be particularly susceptible to the harmful effects of the chemicals. Zinc phosphide is not particularly toxic itself. However as soon as the compound comes into contact with the acid in a rats stomach it breaks down and releases a HIGHLY toxic gas called phosphine.
Phosphine breaks down the cells inside the rats body. The gas is non-specific in how it destroys the rats internal organs but usually the rats die from pulmonary edema when the lungs. Hydrogen Phosphide see Phosphine 73 Hydrogen Sulfide 150 Hydrogen Sulfide Ag Sol 150 Hydrogen Sulfide dry 150 Hydroquinone 150 Hydroxyacetic Acid 150 Hydroxylamine Sulfate 120 Hypochlorous Acid 120 Chemical Substance Concentration TemperatureºF Chemical Substance ConcentrationTemperatureºF Chemical Resistanceof Polypropylene and Polyethylene Acid Waste.
Another mole killer consists of Zinc phosphide is quite famous for its effectiveness. This one comes in pellet form and chemically reacts to phosphine gas. Its a fast action killer and can kill moles within 23 hours.
That is why its marketed as high yield mole and gopher bait. You will get one pound of a package of it. In addition poison peanuts come into this category.
These products include bromethalin neurotoxin cholecalciferol hypercalcemic renal failure and zinc phosphide phosphine gas induced pulmonary edema. The Vitamin K deficiency panel will identify patients with active bleeding due to anticoagulant rodenticides such as warfarin diphacinone and brodifacoum whose mechanism of action is based on vitamin K antagonism. What other diseases.
Hazardous Waste No. How the EPA conducts risk assessment to protect human health and the environment. Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments.
Exempt when present in motor oils at 25 or below. Zinc oxide is exempt except when present as dust or when generated as a fume. Zinc stearate is exempt except when present as dust.
Refers to solutions greater than or equal to 4. Refers to solutions greater than or equal to 3. Refers to any mixture containing 01 or greater.
Only those codes applicable to the University of Maryland are listed Hazardous waste is any solid waste that either exhibits any of the characteristics of hazardous waste or is a listed EPA waste. In addition EPA Hazardous Waste Codes are also classified as acute and non-acute. P-listed codes and certain dioxin codes F020-F023 and F026-F028 are considered to be acute.
Zinc phosphide causes severe irritation if ingested. It reacts with water and stomach juices to release phosphine gas which can enter the blood stream and affect the lungs liver kidneys heart and central nervous system. Zinc phosphide is easily absorbed through the skin or inhaled from fumes.
With repeated exposure it accumulates in the body to dangerous levels. Signs and symptoms of mild. Chemical name Notes Reportable quantity pounds Threshold planning quantity pounds 75-86-5.
Purchase Encyclopedia of Toxicology - 3rd Edition. Print Book E-Book. 10 454 Zinc fluoride.
1000 454 Zinc formate. 1000 454 Zinc hydrosulfite. 1000 454 Zinc nitrate.
1000 454 Zinc phenolsulfonate. 5000 2270 Zinc phosphide Zn3P2. 100 454 Zinc silicofluoride.
Sixteen 11 of 142 exposed casino workers developed TILT. Of 142 pilots and fliers that have reported illness from fumes while flying 16 11 have developed TILT. Eight 27 of the 30 tunnel workers exposed to gas station vapors developed the disease.
In the case of a family living in a moldy home all nine family members became sick but four started to experience TILT symptoms. Beyond Pesticides November 8 2021 California state agencies led by the California Natural Resources Agency CNRA released a draft Natural and Working Lands Climate Smart Strategy to guide and accelerate near- and long-term climate action across key California landscapes. All states need such strategies and to be effective they must be backed by ambitious targets focused on reduction.
Toxicity criteria on chemicals evaluated by OEHHA. OEHHA chemical database meta data Export database as CSV file If you are having trouble with the download and would like a copy of the database just drop me LaurieMonserratoehhacagov a note and I will provide you a csv file.