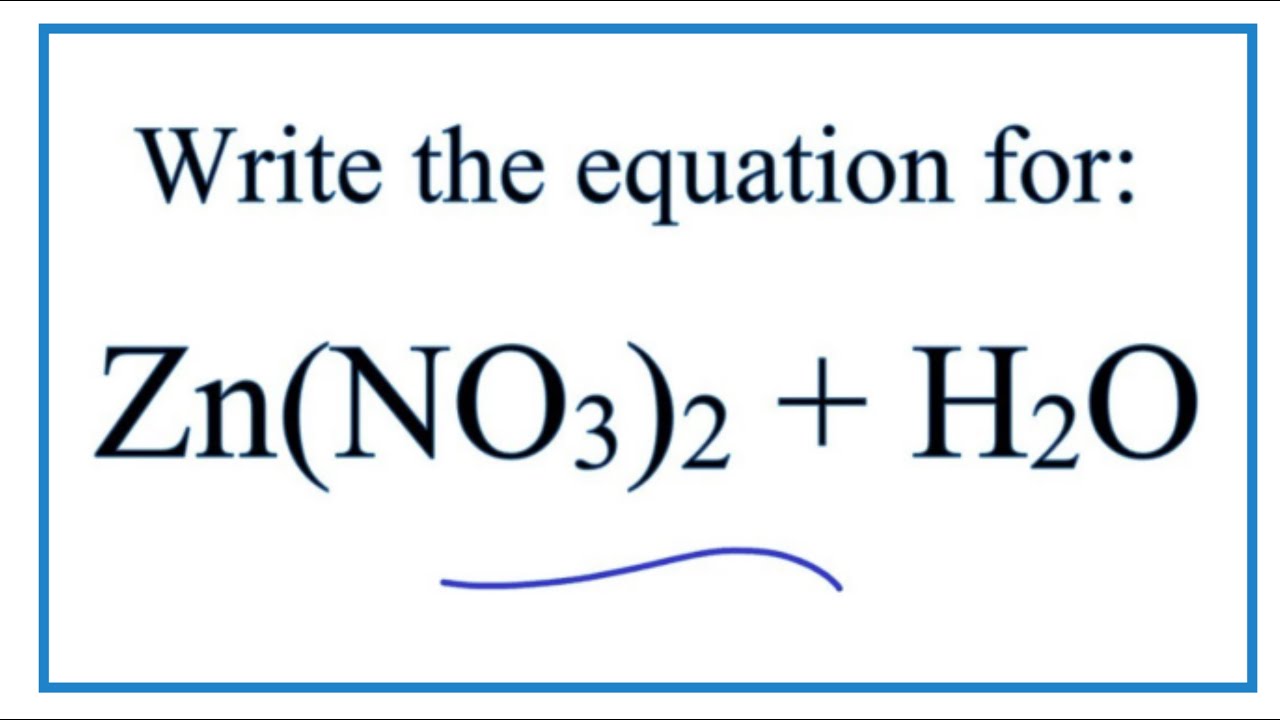
Arsenic Uranium Lead NitrateNitrite Fluoride 5139 Note that the requisition form in the Halifax area and West Hants combine the price of package 2 and bacterial analysis for a total of 8139. From instruments to reagents meters to probes media to general lab supply this handbook outlines everything you need to perform each procedure simplifying the water.
Package 3 Calcium Magnesium Aluminum Boron Barium Beryllium Cadmium Cobalt.
Zinc nitrate in water. Zinc nitrate is an inorganic chemical compound with the formula ZnNO 3 2. This white crystalline salt is highly deliquescent and is typically encountered as a hexahydrate ZnNO 3 2 6H 2 O. It is soluble in both water and alcohol.
Zinc nitrate is usually prepared by dissolving zinc in nitric acid this reaction is concentration dependent with a reaction in. Zinc in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality _____. Cadmium nitrate and nitrite in addition to food additives.
Further up-to-date information on the GDWQ and the process of their development is available on the WHO internet site and in the current edition of the GDWQ. Acknowledgements The work of the following coordinators was. The nitrate ZnNO 3 2 chlorate ZnClO 3 2 sulfate ZnSO 4 phosphate Zn 3 PO 4 2 molybdate ZnMoO 4 cyanide ZnCN 2 arsenite ZnAsO 2 2 arsenate ZnAsO 4 2 8H 2 O and the chromate ZnCrO 4 one of the few colored zinc compounds are a few examples of other common inorganic compounds of zinc.
One of the simplest examples of an organic compound of zinc is the acetate ZnO 2 CCH 3 2. The zinc dust catalyzes the reduction of nitrate to nitrite. A red color should develop in the medium which still contains unreduced nitrate.
It is important however not to add too much zinc dust. Excess zinc dust will catalyze the reduction of nitrite produced from that nitrate resulting in a colorless medium and the incorrect interpretation of the test as positive a false-positive result. Determination of nitrate reduction to nitrite is a two step process.
First the reduction of nitrate to nitrite is determined by the addition of Nitrate Reagents A and B then if necessary the reduction of nitrate beyond nitrite is determined by the addition of Nitrate Reagent C zinc dust. Inoculate the nitrate broths with bacterial suspension. Do NOT use water or foam on burning zinc metal.
Apply dry chemical sand or special powder extinguishing media. Zinc is relatively non-toxic and poses little immediate hazard to the health of emergency response personnel or to the environment in an emergency situation. July 15 2015 Zinc Metal Page 2 of 6 Potential Health Effects.
Zinc is essentially non-toxic to humans. However zinc oxide. Water nitrate and nitrite intake during pregnancy was estimated in a multi-center case-control study of childhood brain tumors in.
Karvonen M Spat Study G. Finnish Childhood Diabetes Registry G. Zinc and nitrate in the ground water and the incidence of Type 1 diabetes in Finland.
The optimal temperature for water is between 65 degrees 18 C and 80 degrees 27 C. Plants grown in colder climates will thrive in cooler water while plants grown in warmer regions prefer warmer water. When you add new water to your reservoir make sure its approximately the same temperature as the existing reservoir water.
06 ppm DTPA extractable Zn Boron. 05 ppm hot water soluble boron Iron. 45ppm DTPA extractable Fe Manganese.
20ppm DTPA extractable Mn Copper. 02ppm DTPA extractable Cu Designed and Developed by National Informatics Centre Information and data in this application is managed by State Agricultural Departments and Department of Agriculture and Farmers Welfare STCR. Nitrate NH 4 Ammonium chloride Ammonium carbonate Ammonium hydroxide Ammonium sulfate Ammonium phosphate Ammonium nitrate KPotassium chloride Potassium carbonate Potassium hydroxide Potassium sulfate Potassium phosphate Potassium nitrate Ca2Calcium chloride Calcium carbonate Calcium hydroxide Calcium sulfate Calcium phosphate Calcium nitrate Zn2Zinc II.
Zinc acetate is designated as a hazardous substance under section 311b2A of the Federal Water Pollution Control Act and further regulated by the Clean Water Act Amendments of 1977 and 1978. These regulations apply to discharges of this substance. This designation includes any isomers and hydrates as well as any solutions and mixtures containing this substance.
Following pretreatment with zinc chloride hepatocytes were treated with 20 or 40 uM silver nitrate for 24 hr and cytotoxicity was then assessed by enzyme leakage and loss of intracellular potassium. The toxicity of silver was significantly less in the zinc-pretreated cells. Magnesium nitrate has a high affinity towards water.
Therefore heating it results to decompose into magnesium oxide nitrogen oxides and oxygen. 2 MgNO 3 2 2 MgO 4 NO 2 O 2. Exposure to Magniosan causes mild irritation in the mucous membranes.
Symptoms include shortness of breath and coughing. Swallowing large doses may result in dizziness vomiting weakness. Run your water.
Before drinking flush your homes pipes by running the tap taking a shower doing laundry or doing a load of dishes. The amount of time to run the water will depend on whether your home has a lead service line or not and the length of the lead service line. Residents should contact their water utility for recommendations about flushing times in their community.
Solids refer to matter suspended or dissolved in the water or wastewater and may affect water or effluent quality in adverse ways. Waters with high dissolved solids generally are of inferior palatability and may induce unfavorable physiological reactions in transient consumers. Solids analyses are important in the control of biological and physical wastewater treatment processes and for.
The Water Analysis Handbook WAH is the result of more than 85 years of research and method development. With over 300 illustrated step-by-step instructions this is your comprehensive source for water analysis procedures. From instruments to reagents meters to probes media to general lab supply this handbook outlines everything you need to perform each procedure simplifying the water.
Im glad you found your way here for chemistry help because youre in the right spot to be enlightened. By the way if its not actually the holidays and I havent updated this recently sorry about that. Here are the rules for using this free website.
I promise that I wont take your. Due to the corrosive and reactive properties of ammonium nitrate and to avoid contamination galvanized iron copper lead and zinc shall not be used in a bin construction unless suitably protected. Aluminum bins and wooden bins protected against impregnation by ammonium nitrate are permissible.
The partitions dividing the ammonium nitrate storage from other products. Zinc Acetate is made from zinc nitrate and acetic anhydride. Zinc acetate is another chemically-altered form of zinc and considered to be more absorbable than gluconate.
This form may aid in reducing the duration of the common cold JRSM Open. 2017 as well as offer relief for Wilsons disease a genetic disorder whereby the body stores toxic levels of copper. Zinc acetate is the best.
Per zinc and some others. An abundance of these minerals can cause hard water plumbing and laundry stains or bad odors. Water testing for lead nitrate and bacteria before mortgage approval.
Contact both the lending institution and the local health department for information on required tests. After a new well is drilled or an existing well is opened for pump repair replacement or any. Amyl Nitrate Strange Connection Dirty Bastard Remix.
Salts for Athletes 100s of Uses per Bottle Explosive Workout Sniffing Salts for Massive Energy Boost Just Add Water to Activate Pre Workout. 1 Count Pack of 1 40 out of 5 stars 2399. 92 1492Count Save more with Subscribe Save.
Get it as soon as Thu Dec 2. FREE Shipping on orders over 25 shipped by Amazon. Simplicity in Water Analysis We provide industrial firms all over the world with water analysis test kits that are accurate and easy to use.
Our customers can depend on us for superior quality not only in our products but in every aspect of their relationships with us including friendly personal service prompt deliveries and well-informed technical support. Nova Scotia Environment recommends that Nova Scotians on well water have it tested for bacteria every. Arsenic Uranium Lead NitrateNitrite Fluoride 5139 Note that the requisition form in the Halifax area and West Hants combine the price of package 2 and bacterial analysis for a total of 8139.
Package 3 Calcium Magnesium Aluminum Boron Barium Beryllium Cadmium Cobalt. It is vitally important that water is sampled and preserved correctly in order to ensure analysis results accurately reflect the in situ parameters analytes and physical water properties that you are trying to measure and base decisions on. Lead Lux Magnesium Manganese Molybdenum Nickel Nitrate Nitrite Nitrogen ORP Ozone pH Phosphate Phosphorus Potassium Refractometry Relative Humidity Resistivity Salinity Silica Silver Sodium salt Sulphate Sulphide Sulphite Sulphur Dioxide Tartaric Acid Temperature Titration Turbidity Zinc.