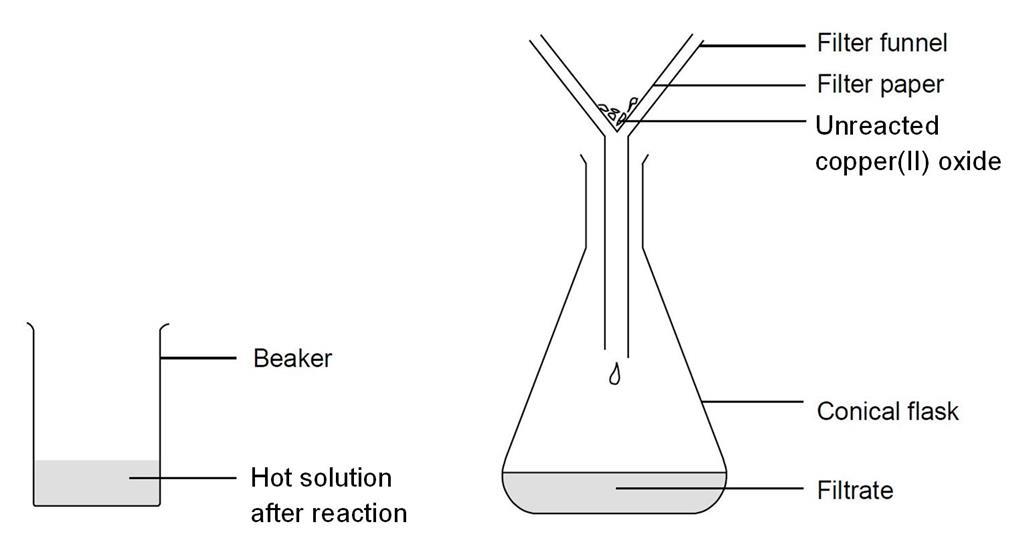
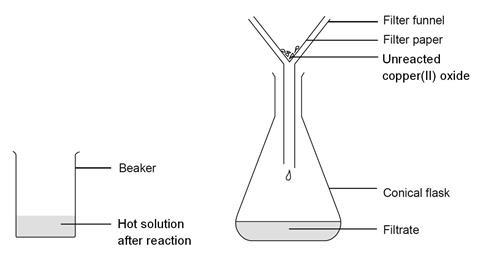
Filter the mixture to remove unreacted copper oxide then heat the filtrate to evaporate off about half the water. The four formulas meet the ever-changing health and breeding requirements of specific herds.
Making hydrated copper sulfate crystals from copper oxide and sulfuric acid requires all of the filtration evaporation and crystallisation techniques.
Why does copper oxide not dissolve in water. But copper oxide is not a metal rather it is a metal oxide. Metal oxides are basic substances that can react with acids to form salt and water. These acid-base reactions are also known as neutralization and are non-redox in nature.
Being a weak base copper oxide reacts with HCL easily to generate a soluble copper chloride and water. Pour the cool residue into a 100 cm 3 beaker and add a little dilute hydrochloric acid to dissolve the zinc oxide and also any unreacted zinc and copper oxide warming if necessary. Red-brown copper will be left.
This can be rinsed with water and passed around the class for observation. Show that the powder conducts electricity using a circuit tester. If further confirmation of identity is.
Sodium oxide and potassium oxide dissolve in water to produce alkalis as follows Na 2 Os H 2 Ol 2NaOHaq K 2 Os H 2 Ol 2KOHaq 42 Science We have observed in Activity 39 that all metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen. Metals such as potassium and sodium react so vigorously that they catch fire.
Sodium oxide and potassium oxide dissolve in water to produce alkalis as follows Na 2 Os H 2 Ol 2NaOHaq K 2 Os H 2 Ol 2KOHaq 42 Science We have observed in Activity 39 that all metals do not react with oxygen at the same rate. Different metals show different reactivities towards oxygen. Metals such as potassium and sodium react so vigorously that they catch fire if.
Copper oxide and filter paper to the acid solution. Stir the mixture with a glass stirring rod until the black solid has completely dissolved. Remove the filter paper from the solution as soon as it is clean using forceps.
Gently rinse the filter paper with 1 - 2 mL of water. Add the rinse to the blue copperII sulfate solution. If residual traces of the CuO remain on the Hirsch funnel.
Each color-coded product is a special formula because beef cow nutrition is not a black-and-white issue. The four formulas meet the ever-changing health and breeding requirements of specific herds. We even balance our macro and trace minerals by region to supplement available forage at different times of the year.
And with our color-coding a quick glance in your mineral feeder tells you if. An electric current is passed through copper. Figure 5 shows the apparatus used.
Figure 5 0 1. 6 Complete the sentence. Choose the answer from the box.
1 mark gas. Shows that copper conducts electricity as a. Choose the answer from the box.
1 mark atoms. However in the presence of water copper is bound to tarnish more quickly. This covers it in copper oxide making the copper surface less reactive and thus lowering its effectiveness.
While it is not harmful to drink water from a copper vessel with tarnish in the short and medium term it should be avoided over a long period. Use only cold water for drinking cooking and making baby formula. Remember boiling water does not remove lead from water.
Regularly clean your faucets screen also known as an aerator. Sediment debris and lead particles can collect in your aerator. If lead particles are caught in the aerator lead can get into your water.
Use your filter properly. If you use a. Copper oxide is a base but it is not an alkali because it is insoluble.
Sodium hydroxide is a base and it dissolves in water so it is also an alkali. Explain why all alkalis. These layers of oxide may be more or less durable in water for instance.
We know that plain carbon steel corrodes faster in water than stainless steel. The difference depends on the composition and the penetrability of their respectively oxide layers. The following description of the corrosion phenomenon will only deal with electrochemical corrosion ie.
Not only does acid rain aggressively dissolve calcium in stone but it corrodes certain types of metal. Vulnerable metals include bronze copper nickel zinc and certain types of steel. A study in the journal Water Air and Soil Pollution by the University of Hong Kong reported that artificial acid rain with a pH of 35 could corrode mild steel galvanized steel stainless steel 304.
Water with a low pH may contain metals like what is already mentioned copper lead and zinc. Drinking water with a pH level over 85 shows that an abnormal state of alkalinity minerals is available. High alkalinity does not represent a health hazard but rather can cause aesthetic issues for example a soluble alkali taste to the water that influences coffee to taste bitter.
Scale develop in. Making hydrated copper sulfate crystals from copper oxide and sulfuric acid requires all of the filtration evaporation and crystallisation techniques. 24 React copper oxide with hot sulfuric acid.
Filter the mixture to remove unreacted copper oxide then heat the filtrate to evaporate off about half the water. Leave the concentrated solution in a warm spot to allow the blue hydrated copper. We will be using one piece to dissolve into the vinegar and peroxide mixture and the other as an electrode later.
The copper scrubby material can be quite sharp - you may want to put on a pair of gloves to do this. Now place the copper into the warm vinegarperoxide mixture. Screw on the lid and gently swirl the liquid and copper in the jar.
As time passes the mixture will become more and. Over time acid water can dissolve copper pipes resulting in blue or green stains on your faucets and fixtures. If you notice this staining its an indication that your plumbing system is becoming eroded.
Left untreated leaks may spring which poses a serious flooding risk. Replacing your homes water system doesnt come cheap which is why its better to prevent acid water from. Glass is a non-crystalline often transparent amorphous solid that has widespread practical technological and decorative use in for example window panes tableware and opticsGlass is most often formed by rapid cooling of the molten form.
Some glasses such as volcanic glass are naturally occurringThe most familiar and historically the oldest types of manufactured glass are silicate. Hot Water Outlet AluminumZincTin anode rods are perfect for use if your water heater does not have a separate anode rod inlet port or your old anode rod is impossible to remove. Contain a built-in heat trap nipple with a fluoroplastic ball that sinks inside the nipple into a seat as water flow stops.
This is designed to keep any cooling hot water from entering back into the water heater. Answer 1 of 11. Simply the acids concentration becomes diluted.
However there is an acid base reaction that Nitric acid is constantly undergoing which is. HNO3 H2O -. Alkali salts are very common and dissolve easily.
Due to the hydroxide ions they produce which increase pH all alkalis are bases. Some sources define any soluble base as an alkali ⁵. As such soluble bases can be described as basic or alkaline.
However insoluble bases such as copper oxide should only be described as basic not alkaline. Alkalinity and the pH of Water. Water is used to dissolve the copper sulphate first and the rest of the ingredients are added to the solution and more water added to make up 1 quart of solution.
Other solutions may also be used depending on the type of iron or steel alloys used in the weapon. The browning process starts as follows. The barrel is first removed of all greasy impurities by washing with soap or detergent.
Dental amalgam is a liquid mercury and metal alloy mixture used in dentistry to fill cavities caused by tooth decay. Low-copper amalgam commonly consists of mercury 50 silver 2232 tin 14 zinc 8 and other trace metals. Dental amalgams were first documented in a Tang Dynasty medical text written by Su Gong 苏恭 in 659 and appeared in Germany in 1528.
Water yes the stuff out of a tap works pretty well. Neutron shielding reflecting and collimating equipment. The neutrons from the source need to pass through the collimator a thing that makes them go in straight lines and the moderator something that slows them down so that they react better and finally into the mercury.
Set up the neutron.