
To ensure freshness and to prevent. HOW TO APPLY - Use daily in the morning or evening for maximum benefit.
Ascorbic acid exists as two enantiomers mirror-image isomers commonly denoted l for levo and d for dextro.
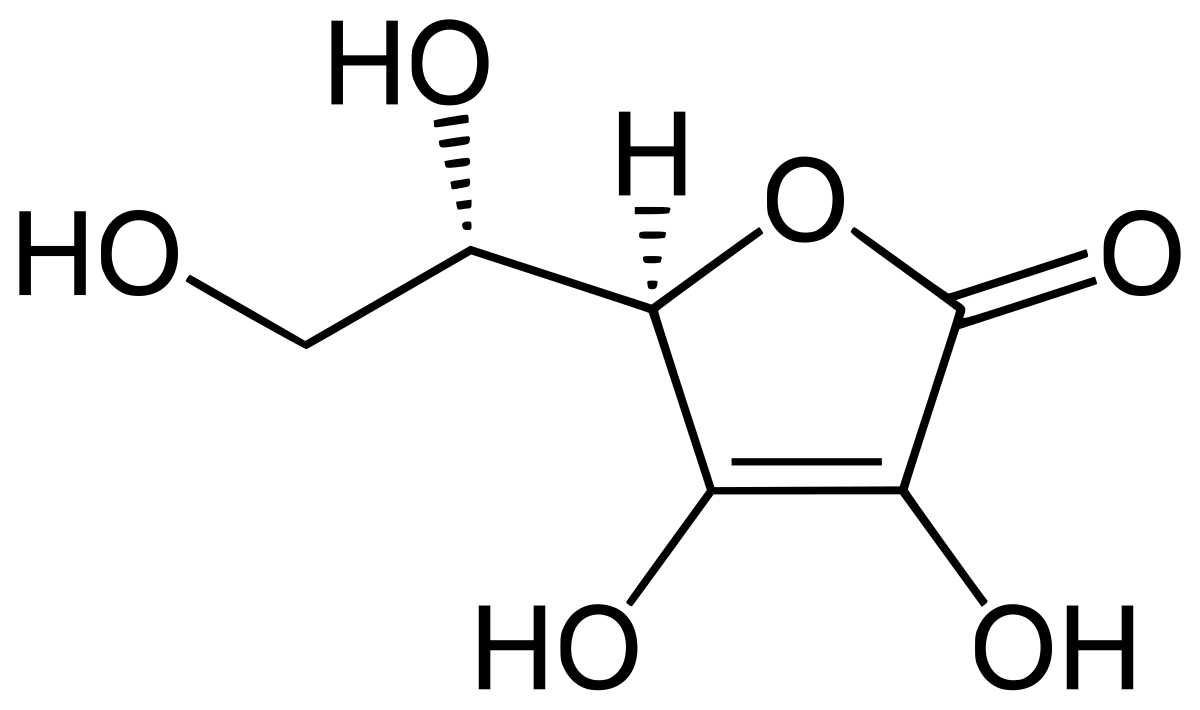
What is the molecular formula of ascorbic acid. Ascorbic acid is an organic compound with formula C 6 H 8 O 6 originally called hexuronic acid. It is a white solid but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions.
It is a mild reducing agent. Ascorbic acid exists as two enantiomers mirror-image isomers commonly denoted l for levo and d for dextro. The l isomer is the one.
Ascorbic Acid is a natural water-soluble vitamin Vitamin CAscorbic acid is a potent reducing and antioxidant agent that functions in fighting bacterial infections in detoxifying reactions and in the formation of collagen in fibrous tissue teeth bones connective tissue skin and capillaries. What is Ascorbic Acid. Ascorbic Acid belongs to monosaccharide family and has a chemical formula C 6 H 8 O 6.
Vitamin C ascorbic acid is a key vitamin to animals and plants. It is a vitamin C and should be obtained in the diet as it cannot be produced by humans. It is also known as Vitamin C or L-.
Vitamin C also known as ascorbic acid and ascorbate is a vitamin found in various foods and sold as a dietary supplement. It is used to prevent and treat scurvy. Vitamin C is an essential nutrient involved in the repair of tissue the formation of collagen and the enzymatic production of certain neurotransmitters.
It is required for the functioning of several enzymes and is important for. Hydrochloric acid helps the body break down foods such as calcium digest and drink them. It also eliminates stomach bacteria and viruses thereby protecting your body from infection.
Stomach acid is composed of hydrochloric acid HCl potassium chloride KCl and sodium chloride NaCl. Hydrochloric acid concentration in the stomach is. Ascorbic acid vitamin C is 4092 C 458 H and 5450 O by mass.
What is the empirical formula of ascorbic acid. C 3 H 4 O 3. What is the empirical formula of each of the following compounds.
Talc by mass composition contains 192 Mg 296 Si 422 O and 90 H. Mg 3 Si 4 O 10 H 34 b. Learn more about VITAMIN C ASCORBIC ACID uses effectiveness possible side effects interactions dosage user ratings and products that contain VITAMIN C ASCORBIC ACID.
Ascorbic acid is reversibly oxidized to dehydroascorbic acid in the body. These two forms of the vitamin are believed to be important in oxidation-reduction reactions. The vitamin is involved in tyrosine metabolism conversion of folic acid to folinic acid carbohydrate metabolism synthesis of lipids and proteins iron metabolism resistance to infections and cellular respiration.
As i said in my fist post the formula I used insisted on including the fact that a small amount of the original Ascorbic acid placed in the solution is NOT in its original state. It is dissociated and hence the denominator in my formula uses the unknown y for the original amount and subtracts off the amount lost by dissociation. This makes for a messy bunch of algebra that I evaded by.
Folate is a B-vitamin that is naturally present in many foods. A form of folate called folic acid is used in dietary supplements and fortified foods. Our bodies need folate to make DNA and other genetic material.
Folate is also needed for the bodys cells to divide. It is important for women to get enough folic acid before and during pregnancy. It can prevent major birth defects of the baby.
The format of this formula is a very fine anhydrous L-Ascorbic Acid powder and as such provides the most direct exposure of extremely high concentrations of Vitamin C topically. With such format a very strong tingling but non-irritating sensation is expected during the first 1-2 weeks of use until the skins tolerance to such high exposure is elevated. To ensure freshness and to prevent.
The van t Hoff factor for ascorbic acid is 1 since it does not ionize in solution it is a weak acid so it actually does ionize a tiny bit but we are ignoring it for this problem 2 molecular weight method 1 1258 mol is to 1 kg as x is to 0250 kg x 03145 mol 550 g 03145 mol 175 gmol. 3 molecular weight method 2 55 g0250 kg 220 g1 kg 220 g is to x as 1258 molal. For example the molecular formula for acetic acid the component that gives vinegar its sharp taste is C 2 H 4 O 2.
This formula indicates that a molecule of acetic acid contains two carbon atoms four hydrogen atoms and two oxygen atoms. The ratio of atoms is 242. Dividing by the lowest common denominator 2 gives the simplest whole-number ratio of atoms 121 so the empirical.
Vitamin C Ascorbic Acid. About 190 C with decomposition Appearance. White to slightly yellowish crystalline powder practically odorless with a strong acidic taste.
The water-soluble vitamin C is probably the most well-known vitamin. Even before its discovery in 1932 physicians recognized that there must be a compound. What is the pseudo formula of ascorbic acid given that ascorbic acid contains 3407 mol of carbon453 mol of hydrogen and 3406 mol of oxygen.
A 010g sample of C_2H_5OH grey. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Aluminum chlorohydrate refers to a group of salts of which the molecular formula given is an example.
Ascorbic acid C 6 H 8 O 6. Washing soda sodium carbonate decahydrate hydrated sodium carbonate C 6 H 8 O 6. Also known as soda ash.
Domestic use as a water softener. Windex ammonia plus detergents dyes and fragrances NH 3 Among other ingredients. Includes a 20 concentration of L-ascorbic acid a highly effective skin antioxidant and collagen builder.
247 PROTECTION - Offering all-day protection against environmental pollutants the lightweight non-greasy formula absorbs quickly and transparently in the skin. Recommended for all skin types. HOW TO APPLY - Use daily in the morning or evening for maximum benefit.
Can be added to any. In some countries this medicine may only be approved for veterinary use. In the US Acetylsalicylic Acid is a member of the following drug classes.
Platelet aggregation inhibitors salicylates and is used to treat Angina Angina Pectoris Prophylaxis Ankylosing Spondylitis Antiphospholipid Syndrome Aseptic Necrosis Back Pain Fever Heart Attack Ischemic Stroke. C 6 H 7 O 6Na or C 6 H 7 O 6 Na or C 6 H 7 NaO 6. L-Ascorbic acid sodium salt.
CID 54670067 Ascorbic acid Component Compounds. CID 5360545 Sodium CID 54670067 Ascorbic acid CID 5785. Hylamide Low Molecular HA or Hyaluronic Acid.
Mineral UV Filters SPF 30 with Antioxidants. The Ordinary Glycolic Acid 7 Toning Solution. Hylamide C25 B Oil.
The Ordinary Skincare Routine For Oily Skin. If you like me have oily skin I bet you want your face to feel fresh all day without that shine that. Infinergy contains approximately 75 caffeine and 25 malic acid by molecular weight.
The malic acid in Infinergy works to buffer the salts in caffeine allowing for easier digestion whilst also replenishing the energy produced by caffeine which helps to minimise the dreaded post-caffeine energy crash. Huperzine is a cognitive enhancer that increases levels of. Drug names in RED are of high risk to all deficiency variants.
Names in GREEN are of low risk. Biosynthesis of coenzyme A CoA from pantothenic acid vitamin B5 is an essential universal pathway in prokaryotes and eukaryotes. COASY is a bifunctional enzyme that catalyzes the 2 last steps in CoA synthesis.
These activities are performed by 2 separate enzymes phosphopantetheine adenylyltransferase PPAT. EC 2773 and dephospho-CoA kinase DPCK. EC 27124 in prokaryotes.
A fatty-acid molecule is composed of a hydrocarbon chain and a carboxyl group formula RCOOH. In saturated fatty acids the carbon atoms are linked together in a chain by single bonds while in unsaturated fatty acids there are one or more double bonds in the hydrocarbon chain see Figure 219. Each glycerol molecule can bind three fatty-acid molecules and as the three need not necessarily.