
Acid oxalic HO O C CO OH. Conjugate bases of strong acids are ineffective bases.
Ethanoic acid is a weak acid which means it does not fully dissociate into ions in water.
What is the ka of benzoic acid. You can derive benzoic acid chemical structure C6H5COOH from benzene by uniting of the water insoluble benzene molecule with a carboxylic acid group -COOH. This produces a water-soluble pleasant-smelling white powder that is used for flavorings and perfume. The formation of benzoic acid has to do with ionizability Water can attach to benzoic acid by hydrogen bonding.
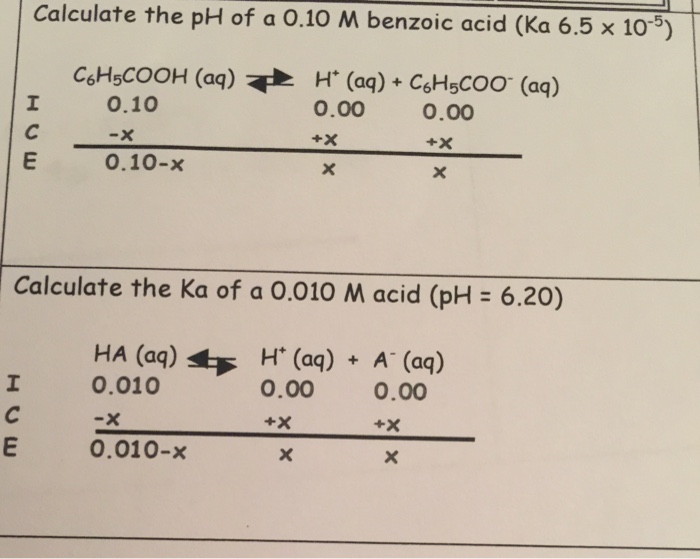
Similarly for the weak acid benzoic acid the reaction would be small value for K HC 7H 5O 2 aq H 2O l H 3O aq C 7H 5O 2 aq In general the equation for the dissociation of the weak acid HA is HA aq H 2O l H 3O aq A aq Since the reaction of a weak acid with water is an equilibrium process an equilibrium expression can be written a K H 3O A. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H 2 O HSO 3 171 x 102 HSO 4 SO 4 2 120 x 102 H 3 PO 4 H 2 PO 4 752 x 103 FeH 2 O 6 3 FeH 2 O 5 OH 2 184 x 103 H 2 C. An acid dissociation constant K a also known as acidity constant or acid-ionization constant.
Hammett originally formulated the relationship with data from benzoic acid with different substiuents in the ortho-and para-positions. Some numerical values are in Hammett equation. This and other studies allowed substituents to be ordered according to their electron-withdrawing or electron.
Terephthalic acid is an organic compound with formula C 6 H 4 CO 2 H 2. This white solid is a commodity chemical used principally as a precursor to the polyester PET used to make clothing and plastic bottles. Several million tonnes are produced annually.
The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid. Terephthalic acid was. C 6 H 5 CO 2 H H C 6 H 5 CO 2- 64 10-5.
STEP 2 Write the Ka expression for the weak acid. STEP 3 Describe each equilibrium concentration in terms of x. X H equilibrium A- equilibrium HA equilibrium HA initial-x.
STEP 4 Assume that the initial concentration of weak acid is approximately equal to the equilibrium concentration. Acid HA A-Ka pKa Acid Strength Conjugate Base Strength Hydroiodic HI I-Hydrobromic HBr Br-Perchloric HClO4 ClO4-Hydrochloric HCl Cl-Chloric HClO3 ClO3- Sulfuric 1 H2SO4 HSO4-Nitric HNO3 NO3-Strong acids completely dissociate in aq solution Ka 1 pKa 1. Conjugate bases of strong acids are ineffective bases.
Hydronium ion H3O H2O 1 00 Iodic HIO3 IO3-16 x 10-1 080 Oxalic 1 -2H2C2O4. 5 Ka 36 x 1013 Explain your choice. Benzoic acid and the benzoate ion is the best choice because the pKa of benzoic acid is closest to 450.
Therefore the resulting buffer will have a base to acid ratio close to one. A buffers job is to prevent large pH changes upon the addition of small amounts of either strong acid or strong base. For an aqueous solution of a weak acid the dissociation constant is called the acid ionization constant Ka.
Similarly the equilibrium constant for the reaction of a weak base with water is the base ionization constant Kb. For any conjugate acidbase pair K_aK_b K_w. Smaller values of pK_a correspond to larger acid ionization constants and hence stronger acids.
Acid strengths decrease down the table a. Conjugate base strengths increase down the table b. Name Acid Conjugate base pK a.
Acetic acid conjugate acid-6. Diethyl ether conjugate acid-35. Consider the titration of 1000 mL of 0220 M benzoic acid Ka 64 10-5 with 0110 M NaOH.
Calculate the pH of the resulting solution after each of the following volumes of NaOH has been added. The acid ionization represents the fraction of the original acid that has been ionized in solution. Therefore the numerical value of K a is a reflection of the strength of the acid.
Weak acids with relatively higher K a values are stronger than acids with relatively lower K a values. Because strong acids are essentially 100 ionized the concentration of the acid in the denominator is nearly. The MW of benzoic acid is 1226 gmol requiring 256 g 26 g using sig fig.
27 a CH3COOHCH3COONa is an acidbase conjugate pair and will form a buffer. B The sodium hydroxide will react completely with the acetic acid to form water and sodium acetate. This is the same acidbase conjugate pair discussed in part a.
12316 Acid dissociation constant Ka 561 pH and acid-base indicators acidity and alkalinity ionization of water 562 Tests for common solutions with acid-base indicators Experiments 1915 Acid-base indicators in the home 80 Acid-base neutralization acid with base forms a salt and water 12111 Butyl chloride rainbow reactions 344 Electric writing sodium chloride with litmus paper. The pH indicates the acidity or basicity of an acid or alkali. The pH scale goes from 0 to 14.
Acids have pH between 0-7. Pure water is neutral and has a pH of 7. Bases and alkalis have pH between 7-14.
The pH can be calculated using. PH -log 10 H. Where H concentration of H ions mol dm-3.
The pH can also be used to calculate the concentration of H ions in solution by. Acid base titration lab answers. The acid equilibrium problems discussed so far have focused on a family of compounds known as monoprotic acidsEach of these acids has a single H ion or proton it can donate when it acts as a Brnsted acid.
Hydrochloric acid HCl acetic acid CH 3 CO 2 H or HOAc nitric acid HNO 3 and benzoic acid C 6 H 5 CO 2 H are all monoprotic acids. Acid benzoic C 6 H 5 CO OH. Acid citric C 6 O 7 H 8 acid formic HC O OH.
Acid lactic CH 3 CH OH CO OH. Acid malic HO O C CH 2 CH OH CO OH. Acid oxalic HO O C CO OH.
Acid piruvic CH 3 CO CO OH. Acid propionic CH 3 CH 2 CO OH. Acid valerianic CH 3 CH 2 3 CO OH.
Ultima editare a paginii a fost efectuată la 8 aprilie 2020 ora 1127. Acest text este disponibil sub. The structural specificity for renal secretion of dicarboxylic acids was revealed by the use of o-phthalic acid and m-phthalic acid as possible inhibitors of TPA secretion.
M-Phthalate but not o-phthalate inhibited TPA excretory transport indicating that there is some specificity in the renal secretion of carboxy-substituted benzoic acids. TPA was actively accumulated by rat and human. Ethanoic acid is a weak acid which means it does not fully dissociate into ions in water.
CH 3 COOH H CH 3 COO-Hydrochloric acid is a strong acid and dissociates fully. HCl H Cl-This means that the concentration of H ions in 04 M HCl is higher than that in 04 M ethanoic acid so its pH is lower and its acid reactions are. Concentrated Solution of Benzoic Acid.
Find the equilibrium concentration of HC 7 H 5 O 2 from a 043 M solution of Benzoic Acid HC 7 H 5 O 2. K_a for HC 7 H 5 O 2 64 x 10-5. The acid dissociation constant pK a is among the most frequently used physicochemical parameters and its determination is of interest to a wide range of research fieldsWe present a brief introduction on the conceptual development of pK a as a physical parameter and its relationship to the concept of the pH of a solution.
This is followed by a general summary of the historical development. MnTBAP Manganese III 5101520-tetrakis4-benzoic acid porphyrin MnTBAP is one of the most intensely explored SOD mimics in biology and medicine 96 97. MnTBAP is an active stable non-toxic and cell permeable SOD mimetic.
Most importantly it possesses both SOD and CAT activity. Academiaedu is a platform for academics to share research papers. After abuse of Copaiva in suppres sion of gonorrhoea or for warts around anus appearing after Copaiva had been used for chancre.
Has been used for dysuria senilis from enlarged prostate in conjunction with Copaiva Inimical. Copaiva Ferrum Zincum met.