
It is colorless odorless non-toxic and highly combustible. Learn more about hydrogen chloride including its properties.
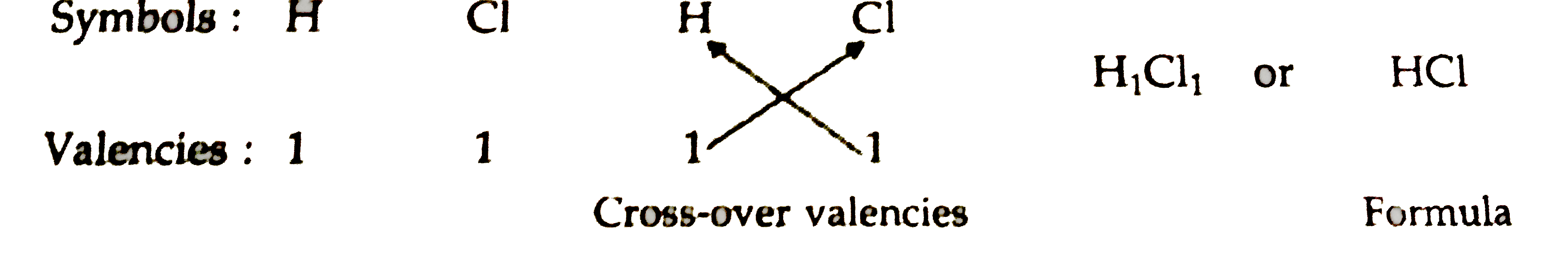
The usual chemical formula for water assumes the hydrogen atoms consist of the isotope protium one proton no neutrons.
What is the formula for hydrogen chlorine. Hydrogen chloride a compound of the elements hydrogen and chlorine a gas at room temperature and pressure. Its chemical formula is HCl. A solution of the gas in water is called hydrochloric acid.
Learn more about hydrogen chloride including its properties. There are two isotopes of chlorine that are stable. They are 37 Cl and 35 Cl.
36 Cl is the stable radioisotope of chlorine. Sodium chloride is the most common compound of chlorine whereas the simplest is hydrogen chloride. Sodium chloride has a molecular formula NaCl whereas hydrogen chloride has a molecular formula HCl.
It is highly reactive. Chlorine and Chlorine Compounds Overview. Hypochlorites the most widely used of the chlorine disinfectants are available as liquid eg sodium hypochlorite or solid eg calcium hypochlorite.
The most prevalent chlorine products in the United States are aqueous solutions of 525615 sodium hypochlorite see glossary usually called household bleach. They have a broad spectrum of. Hydrogen gas forms the simplest covalent bond in the diatomic hydrogen molecule.
The halogens such as chlorine also exist as diatomic gases by forming covalent bonds. The nitrogen and oxygen which makes up the bulk of the atmosphere also exhibits covalent bonding in forming diatomic molecules. It has the mass composition of 1006 of carbon 085 of hydrogen and 8909 of chlorine.
To Determine the Molecular Formula. The compound is an acid having the molar mass of 9808 g mol 1. It has the mass composition of 206 of hydrogen 3269 of sulphur and 6525 of oxygen.
C 9 H 19 NO 4. H 18 N 6 Cl 3 Co. CHC 3 chloroform H.
There are three isotopes of hydrogen. The usual chemical formula for water assumes the hydrogen atoms consist of the isotope protium one proton no neutrons. Heavy water is also possible in which one or more of the atoms of hydrogen consist of deuterium symbol D or tritium symbol T.
Other forms of the chemical formula for water include D 2 O DHO T 2 O and THO. When hydrogen reacts with chlorine hydrogen chloride is formed. Hydrogen chloride is a gas and has the formula HClg.
When hydrogen chloride dissolves in water hydrochloric. The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. This 10-question practice test deals with finding the molecular formula of chemical compounds.
A periodic table will be required to complete this test. Answers appear after the final question. An organochloride organochlorine compound chlorocarbon or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorineThe chloroalkane class alkanes with one or more hydrogens substituted by chlorine provides common examples.
The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names. EXPL THER Hydrogen-rich saline HRS is a novel protection against various oxidative disorders and almost all types of inflammationMoreover its toxicity and side effects are rarely reported. We sought to clarify the protective effect of HRS against the oxygen-induced retinopathy OIR in C57BL6 J modelThe OIR in the HRS treated mice and the untreated controls were systematically compared.
Hydrogen chloride when mixed with water H 2 O forms hydrochloric acid a strong and commercially important acid. Other chlorine compounds include. Chloroform CHCl 3 carbon tetrachloride CCl 4 potassium chloride KCl lithium chloride LiCl magnesium chloride MgCl 2 and chlorine dioxide ClO 2.
Chlorine is a very dangerous. An insecticide is known to contain carbon hydrogen and chlorine. Reactions were carried out on a 1000 gram sample of the compound and converted all of its chlorine into ions dissolved in water.
The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. An ionic compound is composed of a metal and a non-metal. Sodium chloride NaCl and magnesium oxide MgO.
The transfer of electrons between metals and non-metals produces charged particles called ions. Metals lose electrons to produce positve ions called cations. Na Mg 2 Non.
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2.
It is colorless odorless non-toxic and highly combustible. Hydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. Stars such as the Sun are.
Our Clorox 2 Stain Remover Color Booster has hydrogen peroxide as its active ingredient and is in the class of oxygen color-safe or non-chlorine bleach. Compare this to our Clorox Disinfecting Bleach with CLOROMAX which uses sodium hypochlorite as. For example the formula for hydrogen chloride hydrochloric acid is HCl.
For glucose it is C 6 H 12 O 6. Using this formula you can identify the number of atoms of each element that makes up the compound. For HCl there is one atom of hydrogen and one atom of chlorine.
For C 6 H 12 O 6 there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms. Find the relative atomic mass of. A chemical formula shows the symbols of the elements in the compound and the ratio of the elements to one another.
Chemical formulas can be quite simple sometimes as H Hydrogen or it can take a rather complicated form such as CH 3 CH 2 OH ethanol. Though these formulas are found to be very hard to learn and understand very necessary to. In this example we are calculating the empirical formula for mass composition.
The most common form of nylon Nylon-6 contains 6368. Empirical formula is the smallest whole number ratio of moles of each element in a compound. CaCl 2– there is 1 mole of calcium for every 2 moles of chlorine.
Level 1 Simple Empirical formula questions. What is the empirical formula of the following compounds. So reduce the formula if you can molecular formula.
C 2 H 4. C 11 H 22 O 11. C 25 H.
Each molecule of water contains two hydrogen atoms bonded to a single oxygen atom. Pure table salt is a compound made from two elements - sodium and chlorine. The ratio of sodium ions to chloride ions in sodium chloride is always 11.
Pure methane is a compound made from two elements - carbon and hydrogen. The ratio of. What is the Relative Molecular Mass of sodium sulphate.
Sodium sulphate has the formula Na 2 SO 4 - thats two sodiums one sulphur and four oxygens. 2 x 23 46. 4 x 16 64.
So we just add up the masses of all these atoms to get the total mass - the relative. There are seven diatomiceElements. Hydrogen H 2 Nitrogen N 2 Oxygen O 2 Fluorine F 2 Chlorine Cl 2 –Iodine I 2 and Bromine Br 2.
These seven elements are so reactive that they can be found very often bonded with another atom of the same type. Homonuclear diatomic molecules–A homonuclear diatomic molecule consists of two atoms of different elements. The sodium Na and chlorine Cl atoms arrange themselves in a specific pattern to form the cubic salt crystals.
Allotropes A diamond is another good example of a crystal. Diamonds are a crystal form of pure carbon C. The carbon atoms of a diamond are connected in a very compact and structured way.
The carbon atoms found in graphite in pencils have a different crystalline arrangement. A hydrogen ion is lost from one of the ligand water molecules. You will also find variations on its formula.
CrKSO 4 212H 2 O. Cr 2 SO 4 3K 2 SO 424H 2 O. K 2 SO 4Cr 2 SO 4 324H 2 O.
The first of these formulae is just the other ones divided by two and rearranged a bit. Personally I prefer the second one because it is easier to understand what is going on. Since the formula C 4 H 8 has two fewer hydrogens than the four-carbon alkane butane C 4 H 10 all the isomers having this composition must incorporate either a ring or a double bond.
A fifth possible isomer of formula C 4 H 8 is CH 3 CHCHCH 3. This would be named 2-butene according to the IUPAC rules. However a close inspection of this molecule indicates it has two possible structures.