That gives you the complex ion. Brand name for Tris Trishydroxymethylaminomethane Oligo.

Water that has completed a recycling process.
What is the abbreviation for hydrogen chloride. 4 Lower outer quadrant LOQ Lost to follow up. LTFU Left upper outer quadrant. The carbon dioxide can diffuse through cell membranes but hydrogen ions cannot and bicarbonate diffusion is prevented as well which threatens an imbalance which could rupture the alveolar cell.
The bicarbonate ion moves into the blood plasma to exchange itself with chloride ions to diffuse into the red cell with the hydrogen ions. The result is a reduction in the number of hemoglobins with. Metalorganic frameworks MOFs are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one- two- or three-dimensional structures.
They are a subclass of coordination polymers with the special feature that they are often porousThe organic ligands included are sometimes referred to as struts or linkers one example being 14. PHhydrogen ion concentration PIDpelvic inflammatory disease PKUphenylketonuria pmbetween noon and midnight PNSperipheral nervous system poby mouth post posposterior postop PostOppostoperative pp pppostprandial after eating pO2partial pressure of oxygen PPDpurified protein derivative TB test. Abbreviations of weights and measures are pronounced using the expansion of the unit mg milligram and chemical symbols using the chemical expansion NaCl sodium chloride.
Some initialisms deriving from Latin may be pronounced either as letters qid cue eye dee or using the English expansion qid four times a day. The following abbreviation and acronym list containing over 3000 entries was originally donated to TECNET by the Naval Training Systems Command NTSC in Orlando Florida. Apparently it was first posted in January 1993 and the last update was in October 1995.
I downloaded it in February 1997 and initiated its revision including the addition of acronyms adding html the table making it. Adjuvant-Alum aluminum-containing adjuvant AS01 B 3-O-desacyl-4-monophosphoryl lipid A MPL Quillaja saponaria Molina fraction 21 QS-21 cholesterol dioleoyl phosphatidylcholine DOPC disodium phosphate anhydrous potassium dihydrogen phosphate sodium chloride water for injection AS04 3-O-desacyl-4-monophosphoryl lipid A adsorbed onto aluminum as hydroxide salt. The term pH comes from the German word potenz which means power combined with H the element symbol for hydrogen so pH is an abbreviation for power of hydrogen Examples of pH Values of Common Chemicals.
We work with many acids low pH and bases high pH every day. Examples of pH values of lab chemicals and household products include. Difference Between PBS and HBSS PBS vs.
HBSS PBS is the abbreviation for phosphate buffered saline while HBSS is the acronym for Hanks balanced salt solution. These two are forms of a saline solution with certain ingredients or chemicals added to achieve their purposes. Both PBS and HBSS are used in cell biology mostly in molecular biology and biochemistry experiments.
Ingredient in vinegar is the weak acid acetic acid. The structure is draw below the red hydrogen is the acidic hydrogen meaning it will be the only hydrogen involved is this acid-base reaction. The abbreviation for acetic acid is HAc.
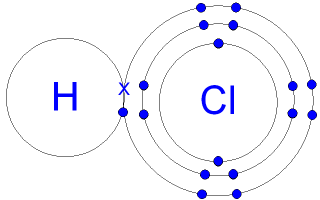
The sodium hydroxide will neutralize the acetic acid HAc in the vinegar. The Ac-actually represents the. Hydrochloric acid abbreviation HCl aq is a common acid both in the body and in the labIt is for example a major component of gastric acid pH 1-2 05 wv HCl.
In experiments it is used among other things to set the pH in buffers eg. Tris and to reveal antigens eg. BrdUChemically speaking it is a solution of the gas hydrogen chloride HCl g in water.
Write the noble gas abbreviation for the following. A 3 1 -1 -12 b atom with five electrons in the 3rd energy level c gallium d potassium e 3. 1 0 -12 Thanks CLASSIFY the primary type of INTERmolecular forces in the compounds they are using in this experiment as ionic dipole dipole hydrogen-bond or Lond.
On DispersionVan der Waals Salt NaCl or sodium chloride ionic. For instance when sodium chloride NaCl dissolves in water the resulting solution contains separated Na and Cl ions. In this equation the s represents the solid state and the aq which is an abbreviation for aqueous shows that the ions are hydratedthat is they have a certain number of water molecules attached to them.
Brand name for Tris Trishydroxymethylaminomethane Oligo. Abbreviation of oligonucleotide or oligomer. Oligonucleotides are short single-stranded DNA or RNA molecules that must be annealed heated or melted so they can bond and form a double strand with an appropriate complementary DNA or RNA strand.
This page discusses the annealing. Because chloride ions are bigger than water molecules you cant fit 6 of them around the central ion - thats why you only use 4. Only one of the 4 lone pairs on each chloride ion is shown.
The other three are pointing away from the copper ion and arent involved in the bonding. That gives you the complex ion. The ion carries 2 negative charges overall.
That comes from a combination of the 2. There are given a list of full forms on different topics. These terms can be categorized in educational organizational finance IT technology science computer and general categories.
Gmol to kgmol Conversion. The abbreviation for gmol and kgmol is gram per mole and kilogram per mole respectively. 1 gmol is 1000 times smaller than a kgmol.
To measure units of measurement are needed and converting such units is an important task as well. Mmol milĭ-mōl one thousandth 10 3 of a mole see mole 1. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen.
This process is represented qualitatively by an. The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids l for liquids g for gases and aq for substances dissolved.
Urea and guanidinium chloride – work by competition These compounds contain functional groups that can accept or donate hydrogen atoms in hydrogen bonding. Picture of structures At high concentration 8 to 10 M for urea and 6 to 8 M for guanidinium chloride they compete favorably for the hydrogen bonds of the native structure. Hydrogen bonds of the alpha-helix will be replaced by.
For severe alkalosis may give ammonium chloride in patients without pre-existing hepatic disease. Administer K chloride for hypokalaemia. It increases blood and urinary pH by dissociation to provide bicarbonate ions which neutralises the hydrogen ion concentration.
The hydrogen as well as the energy to heat the process is generally sourced from natural gas methane. Approximately 65 of the natural gas used in the process is needed as a source of hydrogen for ammonia and the remaining 35 is used for heating the process itself. Due to improving technology the energy efficiency of the process has enhanced over time.
Globally 450 million t of nitrogen. By calculating the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode primary pH standard values are calculated using a concentration cell with transference. A glass electrode and pH metre or colour-changing indicator may be used to measure the pH of aqueous solutions.
PH of Acids and Bases Red Litmus Test. A term used to describe the hydrogen-ion activity of a system. A solution of pH 0 to 7 is acid pH of 7 is neutral pH 7 to 14 is alkaline.
Water that has completed a recycling process. Suitable for drinking US standard 500 ppm Potassium Chloride. Salt used to prepare micromhomicrosiemens standards KCl.
Parts per million. Pressure Reducing Valve water distribution PTE. Rotating Biological Contactor wastewater treatment RBTS.
Reed Bed Treatment System wastewater treatment RO. Reverse Osmosis water.