The High Temperature Shift catalyst began the process of reduction where steam passes through the catalyst bed and out the SP8 vent stack. A base is a substance that will dissolve in water to yield hydroxide ions OH.
General chemistry I need the oxidation half reactions reduction half reactions and net ionic equations for the following reactions.
What does chromium nitrate dissolve in. Does a reaction occur when aqueous solutions of leadII nitrate and chromiumIII chloride are combined. Yes no If a reaction does occur write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Also does NaCl and Pb no3 2 form a precipitate. When PbNO32aq Is Mixed With NaClaq A Precipitate Of. Does a reaction occur when aqueous solutions of chromiumII sulfate and potassium hydroxide are combined.
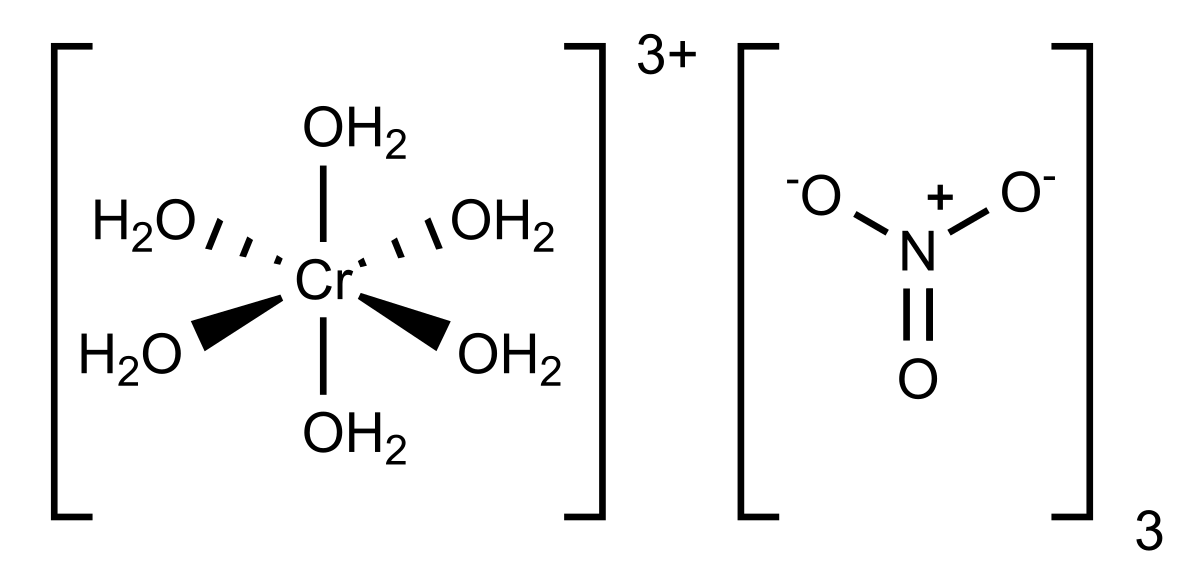
General chemistry I need the oxidation half reactions reduction half reactions and net ionic equations for the following reactions. 1magnesium 01M zinc sulfate 2copper01M zinc sulfate 3zinc01M copperIIsulfate 4zinc3M HCl 5copper3M. The chromium nitrate which during the etching process forms a dark constantly new formation on the chromium layer is very soluble in water and thus in the chromate etchants.
Compatibility and Selectivity All of our photoresists are suffi ciently stable in ceric ammonium nitrate and perchloric acid-based etching mixtures to be used as resist masks. Copper silver and vanadium are strongly. Hexavalent chromium was released from the Newcastle Orica Koorgang Island ammonium nitrate plant on August 8 2011.
The incident occurred when the plant entered the start up phase after the completion of a five-yearly maintenance overhaul. The High Temperature Shift catalyst began the process of reduction where steam passes through the catalyst bed and out the SP8 vent stack. Chromium is a naturally occurring element found in rocks animals plants soil and in volcanic dust and gases.
Chromium is present in the environment in several different forms. The most common forms are chromium0 chromiumIII and chromiumVINo taste or odor is associated with chromium compounds. ChromiumIII occurs naturally in the environment and is an essential nutrient.
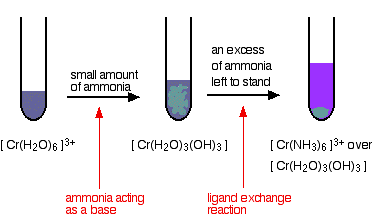
Amphoteric characteristics of chromium compounds. Some 3d metal compounds such as chromium hydroxide chromiumIII oxide ferric oxide has amphoteric characteristics. Amphoteric properties of chromium hydroxide CrOH 3 Chromium hydroxide CrOH 3 is an amphoteric compound and a green precipitateWhen NaOH aq is added that precipitate dissolve and give to CrOH 4 - aq solution.
In what way and in what form does nitrogen react with water. Nitrogen gas does not react with water. It does dissolve in water.
Solubility of nitrogen and nitrogen compounds. Nitrogen N 2 solubility at 20 o C and pressure 1 bar is approximately 20 mgL. Nitrogen solubility may differ between compounds.
Nitrogen I oxide solubility is 12 gL and nitriloacetate salt solubility is 640 gL. The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ip. The distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr.
Nitrate Nitrate is the most. These contaminants have the potential to make their way into groundwater. The migration of metals and metalloids in groundwater will be affected by several factors in particular by chemical reactions which determine the partitioning of contaminants among different phases and species.
Thus the mobility of metals primarily depends on the. The hydroxides of calcium strontium and barium are moderately soluble. Ammonium hydroxide does not exist.
Ammonium hydroxide is a misnomer for aqueous ammonia NH 3 aq. Reaction with nitric acid. Add nitric acid to the compound and observe any reaction that occurs.
If the compound dissolved in water it should dissolve in nitric acid. Does nitrate ion form precipitates with cations. All nitrate compounds are soluble in water.
That means there are no precipitates of nitrate compounds. Also this is same for nitrous ion. NO 2-Questions asked by students.
Ask your question and find the answer free. Which anion will form a precipitate with Ba2. In anaerobic respiration nitrate NO 3- sulfate SO 4 2- metals such as iron Fe 3 and manganese Mn 4 or even CO 2 can play the role of oxygen accepting electrons from the degraded contaminant.
Thus anaerobic respiration uses inorganic chemicals as electron acceptors. In addition to new cell matter the byproducts of anaerobic respiration may include nitrogen gas N. Chromium Cr The major difficulty that I have experienced with Cr is that it often exists in forms that are difficult to put into solution.
Chromite FeOCr 2 O 3 chromic oxide pigments stainless steel and ferro-chrome all present a challenge but the hexavalent chromium oxides are the most difficult. If the oxide has been ignited pigments. Chromium metal can be produced from the high temperature reaction of Cr 2O3 chromiumIII oxide with silicon or aluminum by each of the following reactions.
Cr2O3 2Al 2Cr Al2O3 2Cr2O3 3Si 4Cr 3SiO2 Calculate the number of grams of aluminum required to prepare 133 g of chromium metal by the first reaction. Calculate the number of grams of silicon required to prepare 133 g of. Total dissolved solids TDS comprise inorganic salts principally calcium magnesium potassium sodium bicarbonates chlorides and sulfates and some small amounts of organic matter that dissolve in water.
The TDS concentration is the sum of all filterable substances in water that can be determined gravimetrically. However in most cases. Small amounts of chromium the the form of trivalent chromium Cr 3 are required in the diet.
Chromium assists in the metabolism of glucose but its exact function is not well understood. Chromium deficiency results in mild diabetes and reduced cholesterol levels. This condition is rare in developed countries.
There are a wide variety of foods which are plentiful in chromium such as brewer. Elementary lead does not dissolve in water under normal conditions 20 o C and pressure 1 bar. It may however occur dissolved in water as PbCO 3 or PbCO 3 2 2-.
A well-known example of a water soluble lead compound is lead sugar leadIIacetate which derived its name from its sweet nature. Lead frequently binds to sulphur in sulphide form S 2- or to phosphor in phosphate form PO 4. Resistance to aliphatic aromatic hydrocarbons that dissolve other rubbers.
Exceptionally good resistance to compression set even at high temperatures. Exceptionally good resistance to atmospheric oxidation sun and weather. Enters environment from old mining operations runoff and leaching into groundwater fossil-fuel combustion cement-plant emissions mineral leaching and waste incineration.
Used in metal plating and as a cooling-tower water additive. Chromium III is a nutritionally essential element. Chromium VI is much more toxic than Chromium III and causes liver and kidney damage.
The sampling tube is constructed of chromium molybdenum steel and its length can be anywhere from 3 to 16 ft. The tube is calibrated every 12 inches. The drive head is attached to the top of the tube to prevent the hammer from deforming the tube when it is driven into the ground.
The sampling tip is removable and different tips are available for different types of soils. The drop hammer is. 168 g of calcium nitrate and 1750 g of ammonium fluoride react completely to form calcium fluoride dinitrogen monoxide and water vapor.
Calculate the mass of calcium nitrate present after the reaction is complete. _ 168 g _ 8 g o all reacted none left _ 010 g. It is 33 lower in sodium than table salt and is rich in calcium and magnesium but also contains potassium selenium copper iron zinc manganese and chromium.
It is available in fine ground semi-coarse finishing salt and coarse whole crystal. Celtic and fleur de sel does contain moisture so it is a great candidate for short term storage but I would not store it long term. Total Suspended Solids TSS is defined as a dry-weight of suspended particles that do not dissolve in a sample of water that can be analyzed by a filter trapped by a filter.
It is a water quality parameter that is used to assess the quality of wastewater after treatment in any type of water or waterbody for example ocean water or a wastewater treatment plant. It is listed as a traditional. A base is a substance that will dissolve in water to yield hydroxide ions OH.
The most common bases are ionic compounds composed of alkali or alkaline earth metal cations groups 1 and 2 combined with the hydroxide ionfor example NaOH and CaOH 2. When these compounds dissolve in water hydroxide ions are released directly into the. A base is a substance that will dissolve in water to yield hydroxide ions OH.
The most common bases are ionic compounds composed of alkali or alkaline earth metal cations groups 1 and 2 combined with the hydroxide ionfor example NaOH and CaOH 2. When these compounds dissolve in water hydroxide ions are released directly into the.