Anode dissolves in the electrolyte 4. Iodometry known as iodometric titration is a method of volumetric chemical analysis a redox titration where the appearance or disappearance of elementary iodine indicates the end point.
The dipole moment of CCl 4 however is 0.
What dissolves in carbon tetrachloride. Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water the carbonic acid H2CO3 reacts further with the calcium carbonate. This chemistry is important in understanding how hard water.
Answer 1 of 7. We will get this as the equation NH4Cl H2O —- NH4OH HCl Basically We have NH4 and Cl- as ions And OH- and H and ions from water Thus we get NH4OH and HCl. This page is designed to keep the Mead community Saunders County residents and interested citizens informed about significant activities related to the cleanup and mitigation actions at.
21Calculate the mass percentage of benzene C6H6 and carbon tetrachloride CCl4 if 22 g of benzene is dissolved in 122 g of carbon tetrachloride. 22 Calculate the mole fraction of benzene in solution containing 30 by mass in carbon tetrachloride. 23 Calculate the molarity of each of the following solutions.
A 30 g of CoNO32. 6H2O in 43 L of solution b 30 mL of 05 M H2SO4 diluted. Carbon Tetrachloride Chloroform Acetone Water Electronegativity H.
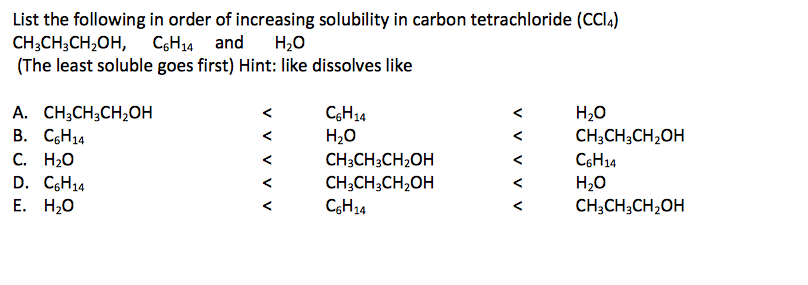
30 Nonpolar Liquids Polar Liquds Figure 1. Nonpolar and Polar Liquids How does polarity difference affect intermolecular forces when two different chemicals interact to form a solution. What does like dissolves like mean.
When dealing with a solid solute and a liquid solvent the solute dissolves. A 2 Bromine in carbon tetrachloride Dissolve 02 g or 02 mL of the compound in 2 mL of carbon tetrachloride or another suitable solvent and add the solution dropwise to 2 ml of 2 bromine solution in carbon tetrachloride and shake. At first glance we might expect a similar dipole moment for carbon tetrachloride CCl 4 which contains four polar C-Cl bonds.
The dipole moment of CCl 4 however is 0. This can be understood by considering the structure of CCl 4 shown in the figure below. The individual C-Cl bonds in this molecule are polar but the four C-Cl dipoles cancel.
Liquids as benzene carbon tetrachloride and petroleum ether have a higher absorption rate with polypropylene than polar media such as ethanol and acetone. Some reduction in tensile strength and an increase in exibility and elongation-to-break in tension can be expected depending on the nature and amount of the organic medium absorbed. HMC PP resins have excellent resistance to.
Not all compounds that contain carbon are organic. An example of an inorganic carbon compound is carbon dioxide. All organics contain both carbon and hydrogen.
A quasi-exception would be carbon tetrachloride which is an organic solvent. Its not actually organic but it is nonpolar so it dissolves organic molecules. Titanium chemical element a silvery gray metal of Group 4 IVb of the periodic table.
It is a lightweight high-strength low-corrosion structural metal and is used in alloy form for parts in high-speed aircraft. Titanium is widely distributed and constitutes 044 percent of Earths crust. Sodium iodide NaI I or C carbon tetrachloride _____CCl4_____I or C ammonia _____NH 3.
Answers will vary but should include statements of like dissolves like. Ionic compounds will be water soluble and molecular compounds will not be water soluble. Based upon the compounds you researched are there any patterns with respect to melting point.
Yes 8 Explain Answers will vary but. Because sugar C12H22O12 molecules are organic by nature due to the presence of carbon in it. But interestingly sugar is insoluble in organic solvents like benzene.
This is because sugar molecules have polarity and require polar solvents to dissolve. Hence we see sugar dissolves well in plain water which is inorganic but having polarity. The figure above shows how the change in vapor pressure that occurs when a solute dissolves in a solvent leads to changes in the melting point and the boiling point of the solvent as well.
Because the change in vapor pressure is a colligative property which depends only on the relative number of solute and solvent particles the changes in the boiling point and the melting point of the. The bond in Carbon Tetrachloride is. Single Covalent Bond 2.
Double Covalent Bond 3. Triple Covalent Bond 1 4. Anode dissolves in the electrolyte 4.
1 Question 17 The observation when ammonium chloride reacts with potassium hydroxide. A reddish brown gas 2. A colourless gas which turns moist red litmus blue.
A green coloured gas which turns. Ozone is slightly soluble in water and much more soluble in inert nonpolar solvents such as carbon tetrachloride or fluorocarbons where it forms a blue solution. At 161 K 112 C it condenses to form a dark blue liquid.
It is dangerous to allow this liquid to warm to its boiling point because both concentrated gaseous ozone and liquid ozone can detonate. At temperatures below 80 K. Write the formula for carbon tetrachloride.
What is avogardos number. How many moles of water H2O contain 201022 molecules of water. And what would the conversion factor be using avocadaos number.
Express the quantity in moles to two significant figures. 20X1022 X1 mol of H2O6022X1023 molecules of H2O 33x10-2 mol. What is the mass of 280 1022 molecules of.
The freezing point constant for carbon tetrachloride is 30. Δt i K f m 44 C 1 30. C kg mol 1 x 150 kg 44 C 1 20.
C mol 1 x x 022 mol 022 mol times 800896 gmol 176 g Ill ignore sig figs and leave it at three. Im such a rebel Problem 2. When 0258 g of a molecular compound benzoic acid was dissolved in 400 g of benzene the.
The compounds include chlorates chloroform synthetic rubber carbon tetrachloride and polyvinyl chloride. Chlorine compounds are used in medicines plastics antiseptics insecticides food paint solvents and many other products. While chlorine is still used in refrigerants the number of chlorofluorocarbons CFCs released into the environment has dramatically declined.
A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3.
If this solution is ideal its total vapor pressure at 20C is. Academiaedu is a platform for academics to share research papers. Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda NaHCO 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry-chemical fire extinguishers.
Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. It is also employed in. Iodometry known as iodometric titration is a method of volumetric chemical analysis a redox titration where the appearance or disappearance of elementary iodine indicates the end point.
Note that iodometry involves indirect titration of iodine liberated by reaction with the analyte whereas iodimetry involves direct titration using iodine as the titrant. Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2. Vinegar is no less than 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements.
Ozone-depleting substances containing chlorine include chlorofluorocarbons CFCs carbon tetrachloride methyl chloroform and hydrochlorofluorocarbons HCFCs. Halons methyl bromide and hydrobromofluorocarbons HBFCs are ODSs that contain bromine. The best-known and most abundant of the ODS are the CFCs.