Antiseptics at low concentrations. Of or relating to one of three possible isomers of a benzene ring with two attached chemical groups in which the carbon atoms with attached groups are separated by two unsubstituted carbon atoms.
A carboxylic acid with two acid groups -COOH.
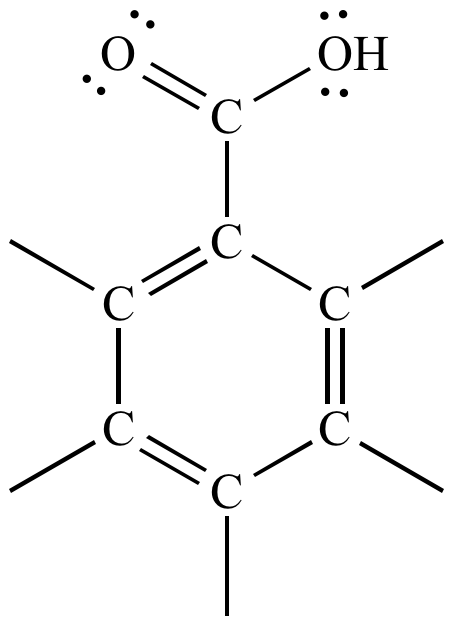
What are the groups in benzoic acid. Benzoic acid BA is a commonly used. Benzoic acid retards the growth of yeast and moulds the effective agent being the undissociated acid. The major food groups contributing to dietary intake of benzoic acid are a wide variety of foods permitted at the following levels.
Various foods 2001000 mgkg prepared salads confectionery etc. Food supplements preserved vegetables. Produced during the bicarbonate extraction compared to some of the other lab groups unknown samples.
A percent composition of 16 benzoic acid supports that there was relatively little benzoic acid compared to the other components. Balmer 7 Answers to Assigned Questions. Page 146 811 8 Fig.
2 shows a flow chart for the separation of benzoic acid 4-nitroaniline and N-4-nitrophenyl. Benzoic Acid is an aromatic acid used in a wide variety of cosmetics as a pH adjuster and preservative. Sodium Benzoate is the sodium salt of Benzoic Acid used as a preservative also in a wide range of cosmetic product types.
Benzyl Alcohol is metabolized to Benzoic Acid which reacts with glycine and excreted as hippuric acid in the human body. Acceptable daily intakes were established by. 4-Aminobenzoic acid also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position is an organic compound with the formula H 2 NC 6 H 4 CO 2 H.
PABA is a white solid although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino.
A carboxylic acid is an organic acid that contains a carboxyl group COOH attached to an R-group. The general formula of a carboxylic acid is RCOOH or RCO 2 H with R referring to the alkyl alkenyl aryl or other groupCarboxylic acids occur widely. Important examples include the amino acids and fatty acidsDeprotonation of a carboxylic acid gives a carboxylate anion.
Synthesis of Benzoic Acid Using the Grignard Reaction INTRODUCTION The Grignard reaction is one of the most general methods for carbon-carbon bond formation in all of organic chemistry. In the first stage of this procedure an organic halide reacts with magnesium metal to form an organomagnesium compound which is known as a Grignard reagent. It is important to recognize that this is.
A carboxylic acid with two acid groups -COOH. A carboxylic acid where the acid group is substituted to one carbon of a benzene ring. An carboxylic acid containing an additional hydroxy group -OH substistuted to another carbon atom than the acid group.
Material Properties - Material properties for gases fluids and. Formic acid and acetic acid are the simplest aliphatic acid and benzoic acid is the simplest aromatic acid. Formic acid and acetic acid are liquids.
Carboxylic acids such as benzoic acid oxalic acid phthalic acid tartaric acid etc are colourless crystalline solids. The following tests can be used to identify carboxylic acids. John Wiley.
Benzoic Acid and Derivatives. Hazardous Substances Data Bank HSDB Primarily in the manufacture of dyes. Kirk-Othmer Encyclopedia of Chemical Technology.
3rd ed Volumes 1-26. John Wiley and Sons 1978-1984 p. V3 790 1978 Hazardous Substances Data Bank HSDB Organic synthesis.
Benzoic acid 4-chloro-p-Chlorbenzoic acid. 4-chlorobenzoic acid is a monochlorobenzoic acid carrying a chloro substituent at position 4. It has a role as a bacterial xenobiotic metabolite.
It derives from a benzoic acid. It is a conjugate acid of a 4-chlorobenzoate. Triclinic crystals or light fluffy.
Of or relating to one of three possible isomers of a benzene ring with two attached chemical groups in which the carbon atoms with attached groups are separated by two unsubstituted carbon atoms. Benzoic acid 80 Benzoic acid o-Nitro-benzoic acid 18 p-Nitro-benzoic acid 2 Di- and Polysubstitution Organic Lecture Series 32 Di- and Polysubstitution Weakly Ortho-para Directing activating Weakly deactivating Moderately activating Strongly activating NH2 NHR NR2 OH NHCR NHCAr OR OCR OCAr R F Cl Br I. Strongly deactivating Moderately deactivating CH OO CR COH SO3 H.
Are acidic due to their OH groups and so will be converted to their ionic salt forms on reaction with an appropriate base. OH O OH OCH 3 H 3CO benzoic acid 2-naphthol p-dimethoxybenzene pK a 417 pK a 95. Note that 14-dimethoxybenzene has no acidic proton and cannot become an ionic salt.
Once an organic compound becomes a salt it possesses a highly polarized area inside its structure. Carboxylic acid - an organic acid characterized by one or more carboxyl groups. Aminobenzoic acid - a derivative of benzoic acid.
Aqua fortis nitric acid - acid used especially in the production of fertilizers and explosives and rocket fuels. Nitrous acid - an unstable inorganic acid known only in solution and as nitrite salts. Aqua regia nitrohydrochloric acid - a yellow fuming corrosive.
Supplier of bulk packaged industrial chemicals. Chemical Products With An Added Ingredient Unmatched Service And Support. As a chemical formulator manufacturer blender and distributor we have the expertise capacity and packaging to meet your needs.
The aryl carboxylate functioned both as substrate and as nucleophile to yield homo-coupled benzoic ester in near-quantitative yield Figure 2 A. Yet the theoretical yield of such transformation is limited to 50 based on the limiting reagent the benzoic acid. To prevent the sacrificial use of half of the substrate we sought to identify an.
As expected the higher the electronegativity of the substituent the greater the increase in acidity F Cl Br I and the closer the substituent is to the carboxyl group the greater is its effect isomers in the 3rd row. Substituents also influence the acidity of benzoic acid derivatives but resonance effects compete with inductive effects. For example benzoic acid is not soluble in water yet it is soluble in sodium hydroxide solution and in sodium hydrogen carbonate solution because these bases react with benzoic acid to form the water-soluble benzoate ion.
The solubility of carboxylic acids and amines is so characteristic that solubility tests alone differentiate these functional groups from all the others in this experiment. Oxalic acid COOH2 - Oxalic acid is the smallest di-carboxylic acid with the chemical formula C2H2O4. Molecular Weight of oxalic acid is 9003 gmol.
Visit BYJUS to understand the properties structure and uses of Oxalic acid C2H2O4 explained by Indias best teachers. Sorbic acid affects yeast growth by inhibiting the uptake of amino acids and the function of sulfhydryl enzymes while benzoic acid destroys the internal proton level of microbial cells. Benzoic acid occurs naturally notably in cranberries cinnamon plums and currants and has been used to inhibit microbial growth for many years including nonalcoholic beverages.
Has covered chemistry for ThoughtCo and About Education since 2001 and other sciences since 2013. She taught chemistry biology astronomy and physics at the high school college and graduate levels. Answer 1 of 3.
The neutralising reaction can produce two salts - either the monosodium variety generally known simply as sodium salicylate where the proton from the carboxylic acid group is donated or the disodium one where both available protons are donated. 120000 new RNA virus species discovered by mining the SRA. Syncmers are better than minimizers Video talks on 16S data analysis posted.
URMAP ultra-fast read mapper paper. 20 of taxonomy annotations in SILVA and Greengenes are wrong Taxonomy prediction is. Inactivates proteins by reacting with sulfide groups.
Disinfectant although occasionally used as an antiseptic on skin. Quaternary ammonium compounds Disrupts cell membranes. Skin antiseptics and disinfectants.
Carbolic acid lysol hexylresorcinol hexachlorophene Denature proteins and disrupt cell membranes. Antiseptics at low concentrations. ALS inhibitors or branched-chain amino acid inhibitors comprise the largest mode of action and include at least one herbicide used in nearly every crop produced in Oklahoma.
Many herbicides in this mode of action fall into two chemical families. Imidazolinones or IMIs or sulfonylureas or SUs but there are three other chemical families within the ALS inhibitors.