005 mm Hg at 25C 77F 10 mm Hg at 107C 224F Solubility in. Safety Data Sheet Material Name.
2 Toluene ratio 41 Toluene makeup 252 due to dehydrogenation losses FeedstockUtilities Toluene.
Toluene vapor pressure. This is caused by a principle called vapor pressure. In chemistry vapor pressure is the pressure that is exerted on the walls of a sealed container when a substance in it evaporates converts to a gas. To find the vapor pressure at a given temperature use the Clausius-Clapeyron equation.
LnP1P2 ΔH vap R1T2 - 1T1. Calculate the vapor pressure of a solution of 740 g of benzene C 6 H 6 in 488 g of toluene C 7 H 8 at 250 C. The vapor pressure of benzene is 951 torr and of toluene is 284 torr at this temperature.
1 Determine moles of benzene and toluene. Benzene — 740 g. Standard ambient temperature and pressure 25C 1013 kPa Disclaimer and references Except where noted otherwise data relate to standard ambient temperature and pressure.
The Physical Property fields include properties such as vapor pressure and boiling point as well. Final AEGLs for Toluene 108-88-3 Exposure Period AEGL-1 AEGL-2 AEGL-3. Toluene ˈ t ɒ l.
J u iː n also known as toluol ˈ t ɒ l. ɒ l-ɔː l-oʊ l is a substituted aromatic hydrocarbonIt is a colorless water-insoluble liquid with the smell associated with paint thinnersIt is a mono-substituted benzene derivative consisting of a methyl group CH 3 attached to a phenyl groupAs such its systematic IUPAC name is methylbenzene. Toluene C 6 H 5-CH 3 is a clear colorless liquid highly flammable with a sweet pungent aromatic odor.
Toluene is less dense than water and is slightly soluble in waterHence it floats on waterToluene vapor is heavier than air. Toluene may be toxic by inhalation ingestion or skin contact. Toluene is present in crude oils and is a product of oil-refining processes thus it is used in.
Toluene is a clear colorless liquid with a distinctive smell. Toluene occurs naturally in crude oil and in the tolu tree. It is also produced in the process of making gasoline and other fuels from crude oil and making coke from coalToluene is used in making paints paint thinners fingernail polish lacquers adhesives and rubber and in some printing and leather tanning processes.
The vapor pressure of a liquid is defined as the pressure exerted by the molecules that escapes from the liquid to form a separate vapor phase above the liquid surface. The pressure exerted by the vapor phase is called the. Vapor or saturation pressure.
Vapor or saturation pressure depends on temperature. If a fluid consist of more than one component a solution components with. Synonyms Trade Names Methyl benzene Methyl benzol Phenyl methane Toluol CAS No.
DOT ID Guide. 1 ppm 377 mgm 3. NIOSH REL TWA 100 ppm 375 mgm 3 ST 150 ppm 560 mgm 3 OSHA PEL TWA 200 ppm C 300 ppm 500 ppm 10.
Temperature K A B C Reference Comment. Besley and Bottomley 1974. Coefficents calculated by NIST from authors data.
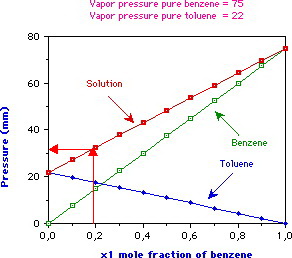
Toluene diisocyanate exists in two isomeric forms 24-toluene diisocyanate and 26-toluene diisocyanate which have similar properties and effectsToluene diisocyanate is produced commercially as an 8020 24-toluene diisocyanate26-toluene diisocyanate mixture of the two isomersAt room temperature the mixture is a clear pale yellow liquid with a sharp pungent odor. At 20 o C the vapor pressures of pure benzene C 6 H 6 molar mass780 gmole and toluene C 6 H 5 CH 3 molar mass920 gmole are 22 mm Hg and 74 mm Hg respectively. What is the total vapor pressure above a solution containing 200 g of benzene and 200 g of toluene at 20 o C.
Structure properties spectra suppliers and links for. Toluene methylbenzene 108-88-3 1262769-46-9 MeC6H5 CH3C6H5. 284 mm Hg 25 deg C Vapor Density.
31 Air1 Evaporation Rate24 Butyl acetate1 Viscosity. 059 cps 20 deg C Boiling Point. 1106 deg C FreezingMelting Point-95 deg C Decomposition TemperatureNot available.
Specific GravityDensity086 Water1 Molecular FormulaC6H5CH3 Molecular Weight9214 Section 10 - Stability and Reactivity Chemical. Catalytic activity and stability of as-obtained MnO x catalysts were evaluated at atmospheric pressure in a quartz fixed-bed reactor and the reaction temperature was measured by a thermocouple located in the centre of catalyst layer as shown in Fig. The total gas flow rate of 50 cm 3 min containing 1000 ppm of toluene was produced from a bubbler using 20 vol O 2 and N 2 as balance.
005 mm Hg at 25C 77F 10 mm Hg at 107C 224F Solubility in. Unspecified Data from Upjohn Company 1970. Return to table Data from IARC 1978.
Return to table Chemical Abstracts. Vapor Pressure is the pressure exerted by a vapor at a given temperature in a closed system A liquid with a high vapor pressure is called a volatile liquid Vapor Pressure is directly related to temperature Increasing Temperature Increased Vapor Pressure Bakken Crude Oil has a Vapor Pressure of 280 360 at 68 F. Specific Gravity Specific Gravity is the weight of a volume.
284 mm Hg 25 deg C Odor threshold. 103 to 140 ugcu m Vapor density. Not Determined Relative density.
0865 gmL at 25 C 77 F MeltingFreezing point. Insoluble in water Boiling pointBoiling range. 110 - 111 C 230 - 232 F Partition coefficient n-octanolwater.
Log Kow 273 Flash point closed cup. 40 C 392 F. Any pressure unit can be used.
The result will be displayed in the same units. The results are only roughly evaluated and can sometimes differ a bit from their real values. Initially the calculator is set to evaluate results for water and substances of similar heat of evaporation DMF aniline toluene.
Allowing for 05 atm pressure drop 985 of MCH condenses at 95 atm and 45C Excess H. And MCH vapor recycled H. 2 Toluene ratio 41 Toluene makeup 252 due to dehydrogenation losses FeedstockUtilities Toluene.
0025 kgkg -MCH Electricity. E kg-MCH Capital Cost 76 million 50000 kg -H. MCH Tank Recycle H.
8 g Heat. A solution of liquid toluene dissolved in liquid benzene has a benzene mole fraction of 850. Calculate the vapor pressure of the solution given that the vapor pressures of pure benzene and toulene are 183 mmHg and 592 mmHg respectively.
The molar quantities of THREE GASES sum to 102mol. What pressure would result if the gases were heated to 301K and confined to a. Pressure MPa bar atm.
Density moll molm 3 gml kgm 3 lb-moleft 3 lbmft 3. Energy kJmol kJkg kcalmol Btulb-mole kcalg Btulbm. Velocity ms fts mph.
Viscosity µPas Pas cP lbmfts. Surface tension Nm dyncm lbft lbin Surface tension values are only available along the saturation curve. Choose the desired type of data.
Data type Isothermal properties Isobaric. Multicomponent Flash Calculation Performs Flash Calculations like BUBBLE P DEW P BUBBLE T DEW T and PT Flash based on Peng Robinson PR Equation of State EOS. Data Number of Components.
First select Component Number then select a Chemical Name. Liquid mixtures having an extremum maximum or minimum vapor pressure at constant temperature as a function of composition are called azeotropic mixtures or simply azeotropes. Mixtures that do not show a maximum or minimum are called zeotropic.
Azeotropes in w hich the pressure is a maximum are often called positive azeotropes while pressure-minimum azeotropes are called negative. Xylenes o- m- p- isomers 1-15. Ethyl 100-41-4.
Safety Data Sheet Material Name. Gasoline All Grades SDS No. 9950 _____ Page 3 of 16 Revision Date 83012 110-54-3.
A complex blend of petroleum-derived normal.