Roughly divided into two categories as mentioned. This article briefly describes major air pollu-tants by presenting their sources dispersion transformation immission concentration of air pollutants in the ambient air and potential effects on health and the environment.

We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer MS-PCET in organic synthesis.
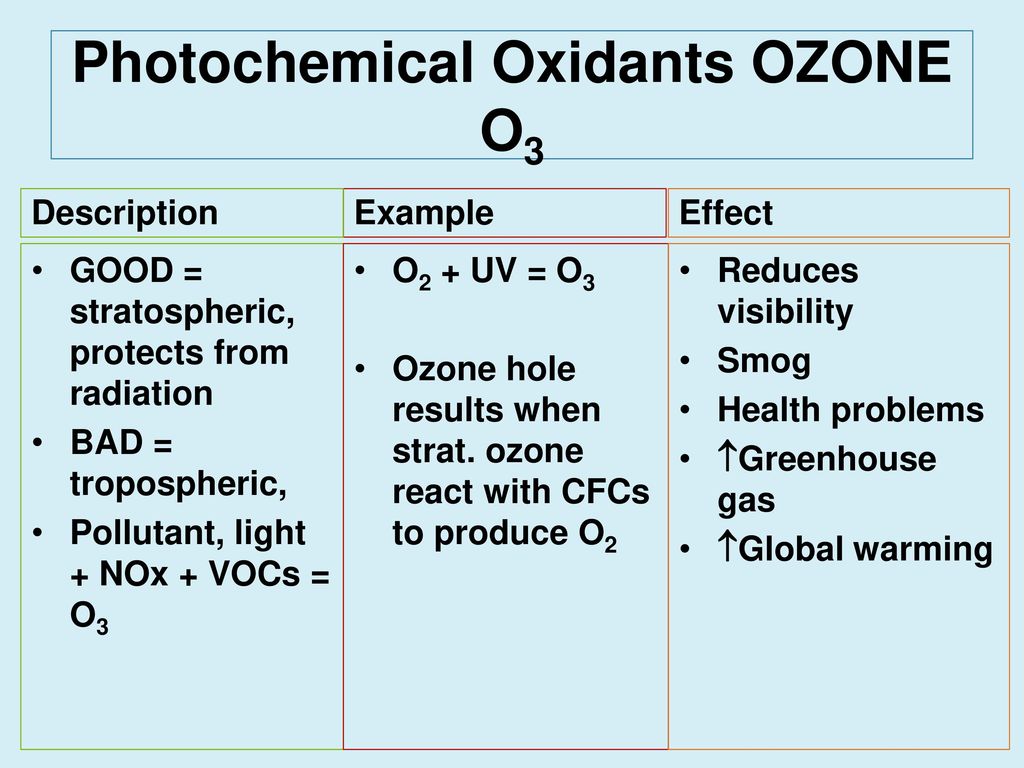
The major photochemical oxidant is. Ground-level ozone constitutes one of the major components of photochemical smog so called because of the photochemical reaction of air pollutants such as nitrogen oxides NOx and volatile organic compounds VOCs with ultraviolet light sunlight. Thus the O 3 levels vary strongly with season and the highest levels of ozone pollution are observed during sunny weather. Oxidant which can generate radicals upon single-electron transfer SET activation of easy-to-oxidize substrates including organic silanes12a If this photochemical SET mechanism could be used to activate allyl silanes 2 too then an allylic radical I would be generated.
A stereocontrolled radical coupling between I and the chiral 5p-electron b-enaminyl radical intermediate III ensuing. Measurements and Lagrangian trajectories constrain a 0-D puff model that approximates plume photochemical history and provides a framework for evaluating key processes. Simulations examine the effects of 1 previously-unmeasured reactive VOC identified in recent laboratory studies and 2 emissions and secondary production of nitrous acid HONO.
Inclusion of estimated unmeasured. Photochemical and free radical reactions are major degradation pathways in the atmosphere so an understanding of the products that are formed is important. The products formed by photochemical reactions may or may not be more toxic than the parent compound.
Once a pesticide for example has been degraded a major removal process for chemicals is to precipitate out of air and return to the. We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer MS-PCET in organic synthesis. MS-PCETs are redox mechanisms in which both an electron and a proton are exchanged together often in a concerted elementary step.
As such MS-PCET can function as a non-classical mechanism for homolytic bond activation providing. Determine the major organic product for the following reaction scheme chegg. Determine the major organic product for the following reaction scheme chegg.
Photostability studies of drugs and drug products are an integral part of the product development process in the pharmaceutical industry. These studies are carried out to ensure quality efficacy and safety of the formulated products during manufacture storage and use. This review deals with the concept of photostability and related aspects and the literature available in the field.
Singlet oxygen is generated by photochemical events whereas peroxyl radicals are produced by lipid peroxidation. These oxidants contribute to aging and degenerative diseases such as cancer and atherosclerosis through oxidation of DNA proteins and lipids. Antioxidant compounds decrease mutagenesis and carcinogenesis by inhibiting oxidative damage to cells.
These vectorial reactions Witt received numerous awards and of Lou Duysens and of Bessel Kok led to a together with associated proton uptake and honours and more than 200 publications scheme with two photochemical reaction release at opposite sides of the membrane as carry his name. His co-workers and former centres in series. At photosystem II electrons hypothesized earlier by Peter.
About the Societies. The Association for Academic Surgery is widely recognized as an inclusive surgical organization. The impetus of the membership remains research-based academic surgery and to promote the shared vision of research and academic pursuits through the exchange of ideas between senior surgical residents junior faculty and established academic surgical professors.
1971 and carbon dioxide and photochemical oxidant emissions limits in 1973. As a result atmospheric and water pollution levels were improved significantly in a relatively short period of time. 1-3-4 Pollution Control Measures Lose Momentum Increased Awareness of.
A new process uses CO 2 as a waste-free terminal oxidant for oxidising alkenes to epoxides. Simultaneously it yields CO. Nonthermal gas plasma has previously been used as an effective technology for splitting CO 2 as well as molecular oxygen and holds some advantages over electro- and photochemical processes.
Low temperature plasmas as the one used in this work are ionised gases. Islam T McConnell R Gauderman WJ Avol E Peters JM and Gilliland F. Ozone oxidant defense genes and risk of asthma during adolescence.
Am J Respir Crit Care Med. Salam MT Millstein J Li YF Lurmann FW Margolis HG Gilliland FD. Birth outcomes and prenatal exposure to ozone carbon monoxide and particulate matter.
This article briefly describes major air pollu-tants by presenting their sources dispersion transformation immission concentration of air pollutants in the ambient air and potential effects on health and the environment. Sources of air pollution Sources of air pollution 1 include natural sources 2 and human-made sources 3. Natural sources Naturally.
The major advantage of using fullerenes as medical antioxidant is their ability to localize within the cell to mitochondria and other cell compartment sites where in diseased states the production of free radicals takes place. Experiments on rats done by Najla Gharbi and coworkers proved this remarkable trait. They showed that aqueous C60 suspensions prepared without using any polar organic.
Wildfires are increasing in size across the western US leading to increases in human smoke exposure and associated negative health impacts. The impact of biomass burning BB smoke including wildfires on regional air quality depends on emissions transport and chemistry including oxidation of emitted BB volatile organic compounds BBVOCs by the hydroxyl radical OH. It is a strong oxidant 52 stronger than chlorine.
It arises in the stratosphere but it could also arise following chain reactions of photochemical smog in the troposphere 63. Ozone can travel to distant areas from its initial source moving with air masses 64. Nitrogen dioxide is a chemical compound with the formula NO 2It is one of several nitrogen oxides.
NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizersAt higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity. These results suggested the concept of two photochemical systems.
The reaction center pigments of PSII and PSI P680 and P700. The linkage of electron transfer and CO 2 assimilation was suggested by studies on Hill oxidant Hill 1937. A linear electron transport system with two light-driven reactions Z scheme was proposed based upon observations of the redox state of cytochromes Hill.
Volved major antioxidant capacity assays can be. Roughly divided into two categories as mentioned. By Huang et al.
2005 as shown in Fig. A number of assays have been developed for. As a major component.
The terminal oxidant in cellular respiration. The ground state of dioxygen is known as triplet oxygen 3 O 2 because it has two unpaired electrons. The first excited state singlet oxygen 1 O 2 has no unpaired electrons and is metastable.
The doublet state requires an odd number of electrons and so cannot occur in dioxygen without gaining or losing electrons such. We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer MS-PCET in organic synthesis. MS-PCETs are redox mechanisms in which both an electron and a proton are exchanged together often in a concerted elementary step.
As such MS-PCET can function as a non-classical mechanism for homolytic bond activation. Water reddish-brown in colour and a strong oxidant. Nitrogen dioxide is an important atmospheric trace gas not only because of its health effects but also because a it absorbs visible solar radiation and contributes to impaired atmospheric visibility.
B as an absorber of visible radiation it could have a potential direct role in global climate change if its concentrations were to become. POCP Photochemical oxidant creation potential worldsteel World Steel Association. Executive summary 11 Introduction Selecting the most appropriate materials for any application depends on the consideration of a range of technical and economic factors including for example functionality durability and cost.
A further and increasingly important factor for material specifiers in a world. Analyze the sources photochemical transformations meteorology and deposition of the chemical species that control the relationships among volatile organic compounds VOCs NO x and ozone in the major urban centers of the United States.