EPAs Emergency Operations Center. EPA also provides additional response assistance when state and local first responder capabilities have been exhausted or when additional support is requested.
Nitrogen N2 CID 947 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards.
The chemical formula for nitrogen. A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols numbers and sometimes also other symbols such as parentheses dashes brackets commas and plus and minus signs. These are limited to a single typographic line of symbols which may include. The chemical formula of a compound is a symbolic representation of its chemical composition.
Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules. For example the chemical formula of water which is H. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.
An ionic compound is composed of a metal and a non-metal. Sodium chloride NaCl and magnesium oxide MgO. The transfer of electrons between metals and non-metals produces charged particles called ions.
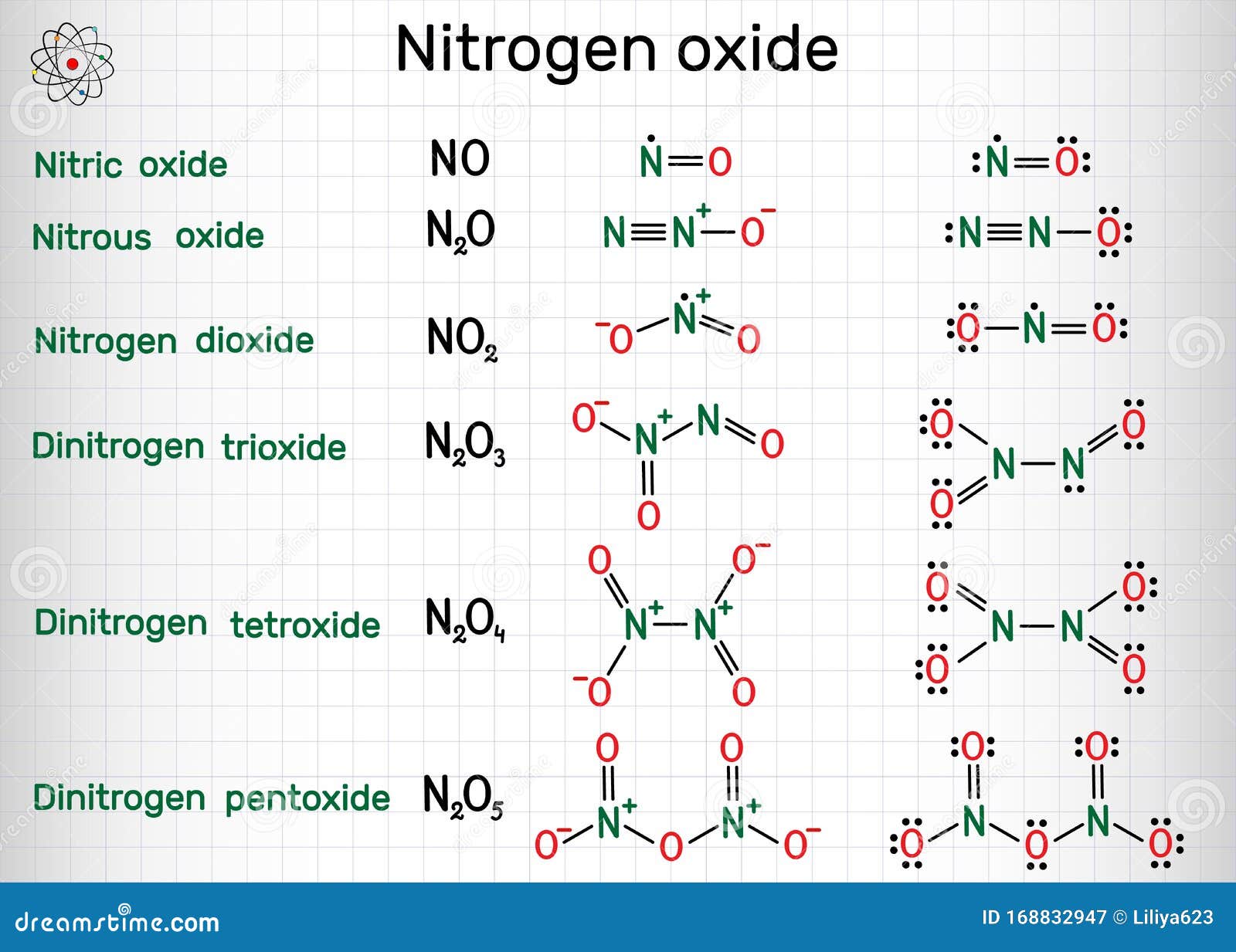
Metals lose electrons to produce positve ions called cations. Na Mg 2 Non. NO 2 is a highly poisonous gas with chemical name Nitrogen dioxide.
It is also called Nitrogen IV oxide or Deutoxide of nitrogen. It is one of the major atmospheric pollutants that absorb UV light and stops to reach it to the earths surface. Nitrogen IV oxide is a yellowish-brown liquid in its compressed form or reddish-brown gas.
Chemical compounds are formed by the joining of two or more atoms. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms.
The two extreme cases of chemical bonds are. Bond in which one or more pairs of electrons are shared. A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance.
Based on the chemical formula of a substance we know the composition of the substance. A The elements that make up the substance. B The ratio or number of atoms of each element in the substance.
Nitrogen molecular weight. Molar mass of N 140067 gmol. Convert grams Nitrogen to moles or moles Nitrogen to grams Percent composition by element.
Atomic Mass of Atoms. 100000 Calculate the molecular weight of a chemical compound. Enter a chemical formula.
Browse the list of common chemical compounds. Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772.
Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean. The best way to learn formula writing is to practice with lots of examples.
Use examples in your chemistry book or look for practice sets online. Do as many as you can until you feel comfortable writing chemical formulas. Symbol for calcium is Ca and symbol of nitrogen is N.
Ca is a group 2 element and has a charge of 2. Nitrogen N2 CID 947 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. A chemical formula is a shorthand method of representing the elements in a compound.
The formula shows the formulas of the elements in the compound and the ratio of the elements to one another. For example the forumla for sodium chloride. NaCl tells us that the compound is composed of the elements sodium Na and chlorine Cl in a one-to-one ratio.
That is one atom of sodium combines with. Dinitrogen tetroxide Nitrogen peroxide Yellowish-brown liquid or reddish-brown gas above 70F with a pungent acrid odor. In solid form below 15F it is found structurally as N₂O₂.
The chemical name of urea is carbamide the diamide of carbonic acid. Its formula is H. Its formula is H 2 NCONH 2.
Urea has important uses as a fertilizer and feed supplement as well as a starting material for the manufacture of plastics and drugs. It is a colourless crystalline substance that melts at 1327 C 271 F and decomposes before boiling. Urea is the chief nitrogenous end.
Writing the chemical formula of compounds requires identifying chemical symbols understanding numbers in formulas and recognizing key prefixes and suffixes. Prefixes like bi- and tri- help identify the number of ions in a molecule. Chemical symbols of elements in the chemical formula represent the elements present and subscript numbers represent mole proportions of the proceeding elements.
Note that no subscript number means a subscript of 1. From a chemical point of view an element contained in the substance is a fundamental question and we represent the elemental composition by a chemical formula such as H2O for. This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript.
For example most samples of the elements hydrogen oxygen and nitrogen are composed of molecules that contain two atoms each called diatomic molecules and thus have the molecular formulas H 2 O 2 and N 2 respectively. Other elements commonly found as diatomic molecules are fluorine F 2 chlorine Cl 2 bromine Br 2 and iodine I 2. The most common form of the element sulfur is.
Urea is a nitrogenous compound containing a carbonyl group attached to two amine groups with osmotic diuretic activity. In vivo urea is formed in the liver via the urea cycle from ammonia and is the final end product of protein metabolism. Administration of urea elevates blood plasma osmolality resulting in enhanced flow of water from tissues including the brain cerebrospinal fluid and eye.
Nitrogen is a chemical element with atomic number seven and atomic weight 14. Its symbol is N. With these facts about nitrogen let us learn about its chemistry physical properties atomic mass and much more.
26 Facts about Nitrogen. It has a melting point of 20986 C 3458 F and a boiling point of 1958 C 3204 F. Nitrogen which is a non-metal was named.
When you write a chemical formula there is a specific order of the elements. In 1900 Edwin A. Hill devised a system of writing a chemical formula that is used for a large number of compounds today.
The Hill system states carbon atoms are listed first hydrogen atoms next and then the number of all other elements in alphabetical order. There are numerous exceptions to this system such as the. Waters chemical formula is H2O this means it is composed of 2 Hydrogen atoms and 1 Oxygen atom.
To find the molar mass find the atomic mass of all the components of a chemical. You can either memorize it or find all of the atomic masses located on the periodic table of elements. In this case hydrogen has an atomic mass of 1 and oxygen has.
The elements with a 2 in their formula are hydrogen nitrogen and oxygen plus the elements in group 7 IUPAC group 17. Formulae of simple covalent compounds A compound contains two or more. EPA responds to oil spills chemical biological radiological releases and large-scale national emergencies.
EPA also provides additional response assistance when state and local first responder capabilities have been exhausted or when additional support is requested. EPA and Emergency Response EPAs Role in Emergency Response. EPAs Emergency Operations Center.
Nitric acid colorless fuming and highly corrosive liquid that is a common laboratory reagent and an important industrial chemical for the manufacture of fertilizers and explosives. It is toxic and can cause severe burns. Learn more about the properties and uses of nitric acid in this article.
Powered by FlexBook textbook Platform CK-12 Foundation 2021.