The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. 1 A T A C C B T U C C C U A C C D U T C C.

The un-ionized form is usually lipid soluble lipophilic and diffuses readily across cell membranes.
Taking aspirin with bases and acids. Taking notes is a very important skill to learn. Do you like sour sweets such as sour worms. The sour taste comes from fumaric acid.
Fumaric acid is a natural acid with a sour taste that is often added to foods. The juice of lemons is rich in ascorbic acid vitamin C and citric acid which makes it taste sour. All acids taste sour.
Does this mean that all acids are safe to taste. Many acids and bases are weak. That is they do not ionize fully in aqueous solution.
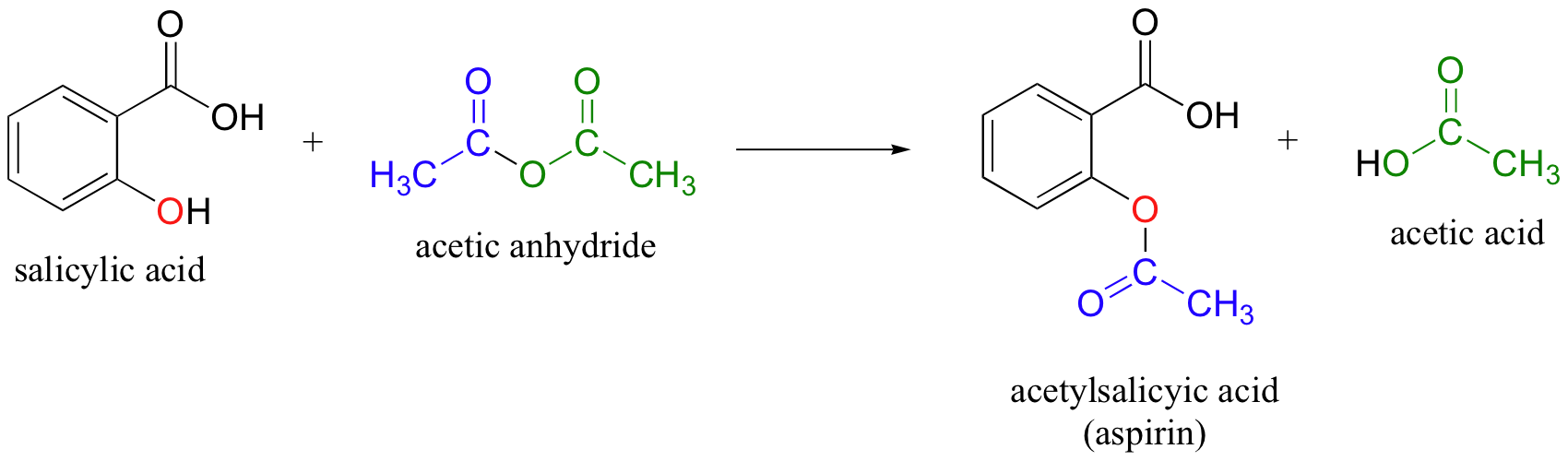
A solution of a weak acid in water is a mixture of the nonionized acid hydronium ion and the conjugate base of the acid with the nonionized acid present in the greatest concentration. Thus a weak acid increases the hydronium ion concentration in an aqueous solution but not as much as the same amount of a. Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation.
Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever. Aspirin given shortly after a heart attack decreases the risk of death. Aspirin is also used long-term to help prevent further heart attacks ischaemic.
Many acids and bases are weak. That is they do not ionize fully in aqueous solution. A solution of a weak acid in water is a mixture of the nonionized acid hydronium ion and the conjugate base of the acid with the nonionized acid present in the greatest concentration.
Thus a weak acid increases the hydronium ion concentration in an aqueous solution but not as much as the same amount of a. The kidneys help the lungs maintain acid-base balance by excreting acids or bases into the blood. The kidneys effect on acidity works much more slowly than that of the lungs.
The relative acceptor strength of Lewis acids toward a series of bases versus other Lewis acids can be illustrated by C-B plots. Aspirin is used as a pain killer and for bringing down fevers. Acids play important roles in the human body.
The hydrochloric acid present in the stomach aids digestion by breaking down large and complex food molecules. Amino acids are. 513 Acids bases and buffers ni pH titration curves for combinations of strong and weak acids with strong and weak bases including.
I sketch and interpretation of their shapes nii pH titration curves for combinations of strong and weak acids with strong and weak bases including. Ii explanation of the choice of suitable indicators given the pH range of the indicator. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal.
Since cross reactivity including bronchospasm between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients meloxicam should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with. In particular people taking fish oil or long-chain omega-3 fatty acid EPA and DHA supplements in combination with anticoagulant drugs including aspirin clopidogrel Plavix dalteparin Fragmin dipyridamole Persantine enoxaparin Lovenox heparin ticlopidine Ticlid and warfarin Coumadin should have their coagulation status monitored using a standardized prothrombin time assay. Theories of acids and bases.
81 A BrønstedLowry acid is a protonH donor and a BrønstedLowry base is a protonH acceptor Amphiprotic species can act as both BrønstedLowry acids and bases A pair of species differing by a single proton is called a conjugate acid-base pair Properties of acids and bases. The bodys maintenance of a healthy pH range for blood and tissues that is slightly basic pH between 735 745. This balance is achieved through the use of systems in the blood which help to minimize pH changes and by the lungs and kidneys which eliminate excess amounts of.
They help to control the balance of acids and bases in your body. The anion gap value is the difference between the negatively and positively charged electrolytes. If the calculated value for the.
In conclusion although the clinical effect of DDIs between NSAIDs and aspirin is unclear clinicians may wish to counsel patients taking a daily aspirin to avoid chronic use of ibuprofen which may reduce aspirins ability to prevent CV events92 The FDA recommends taking ibuprofen 8 hours before or 30 minutes after immediate-release not enteric-coated of aspirin to reduce the. Meloxicam is a pastel yellow solid practically insoluble in water with higher solubility observed in strong acids and bases. It is very slightly soluble in methanol.
Meloxicam has an apparent partition coefficient log Papp 01 in n-octanolbuffer pH 74. Meloxicam has pKa values of 11 and 42. Acids bases and strongly polar compounds often produce streaks rather than spots in neutral solvents.
Streaks make it difficult to calculate an R_f an d may occlude other spots. Adding a few percent of acetic or formic acid to the solvent can correct streaking with acids. Similarly for bases adding a few percent triethylamine can improve results.
For polar compounds adding a few. The opening essay of chapter 12 acids and bases improperly explains the physiological danger of bases as only needing an H ion compared to acids needing an OH- ion. At the end of the chapter the irritating effect of aspirin to the stomach lining due to its mild acidity is overstated.
Most drugs are weak organic acids or bases existing in un-ionized and ionized forms in an aqueous environment. The un-ionized form is usually lipid soluble lipophilic and diffuses readily across cell membranes. The ionized form has low lipid solubility but high water solubilityie hydrophilic and high electrical resistance and thus cannot penetrate cell membranes easily.
Ulcer located anywhere in esophagus stomach or duodenum. In stomach are known as gastric ulcers. All begin with irritation of mucous membranes caused by hydrochloric acid that strips away the protective mucus from surface of mucous membrane.
Action of pepsin protein-digesting enzyme begins to break down underlying membrane. When any of these happen chemical reactions and processes in your body dont work right. Although severe episodes can be life-threatening sometimes metabolic acidosis is a mild condition.
Most drugs are weak acids or bases and as such. Conversely alkalinization of the urine is used to increase the excretion of acetylsalicylic acid aspirin an acidic drug. Increasing the pH of urine above the pK a of acetylsalicylic acid increases the proportion of the drug in the ionized state by about 10000 times.
The ionized form of the drug is not able to be reabsorbed across the. Explain that the orders of bases in DNA and of amino acids in proteins are used as a more accurate means of classification Explain that organisms which segment a more recent ancestor are more closely related have base orders in DNA that are more comparable than those that share only a distant ancestor. Features of organisms.
Core For these IGCSE Biology past year papers you. Evidence-based research provides the basis for sound clinical practice guidelines and recommendations. The database of guidelines available from the National Guideline Clearinghouse and the recommendations of the US.
Preventive Services Task Force are especially useful. 3 The diagram below shows the sequence of bases in a short length of mRNA. A U G G C C U C G A U A A C G G C C A C C A U C a i Place a cross in the box next to the letter that shows the DNA sequence which is complementary to the first four of these bases.
1 A T A C C B T U C C C U A C C D U T C C. The release of cellular nucleic acids and purine nucleotides by these drugs induces uric acid production and may lead to markedly increase serum uric acid levels with acute uric acid nephropathy in some cases. Hyperuricaemia induced by cytotoxic drugs is the most serious type of drug-induced hyperuricaemia.
It usually develops 4872 h after cytotoxic therapy. TLS induces a higher mortality. Most chemistry curriculums are comprised of thick boring textbooks that require a firm grounding in algebra and feature labs with chemicals that can strike fear in a homeschool parents heart.
Our chemistry curriculum is like nothing out there in the homeschool market that I know of. You dont need to know any higher level math to do it. You dont have to purchase a 250 lab kit with.