Assume that there is no volume change when adding the grams of NaOH. Not to mention a shrinking or flattening population brings along its own issues to solve.

Hang the ignition tube in the flask carefully.
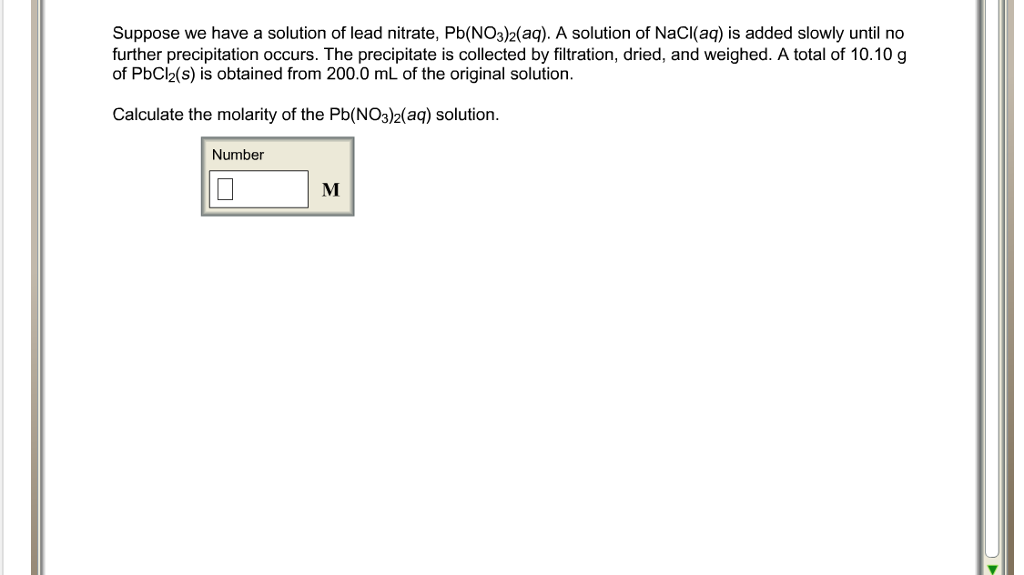
Suppose we have a solution of lead nitrate. For example suppose you have a near-boiling solution of potassium nitrate in water. Well take a solution containing 100 g of potassium nitrate and 100 g of water. Now let the solution cool.
At all temperatures above that marked on the graph about 57C 100 g of water will dissolve more than 100 g of potassium nitrate. All the potassium nitrate will stay in solution. At 57C you hit the.
To ensure that there is no longer Pb 2 is useful an indirect assay. The washing water that we examine is added with some drops of silver nitrate AgNO 3If there is the Pb 2 ion will also be present the chloride ion indeed its concentration will be at least double given that the lead-chloride ratio in the chloride lead is 12. So if there is Pb 2 and then chloride Cl-ions in significant.
46 We have seen that ions in aqueous solution are stabilized by the attractions between the ions and the water molecules. Why then do some pairs of ions in solution form precipitates. Section 42 47 Which of the following ions will always be a spectator ion in a precipitation reaction.
A Cl b NO 3 c NH 4 d S 2 e SO 4 2. Section 42 48 The. Suppose a solution is prepared by dissolving 150 g NaOH in 0150 L of 0250 M nitric acid.
What is the final concentration of OH ions in the solution after the reaction has gone to completion. Assume that there is no volume change when adding the grams of NaOH. Commercially available HCl is 350 ww and has a density of 120 gmL.
Suppose 1750 mL was diluted to 100 L with water to make a more dilute HCI solution. A dry mixture of KOH K2CO3 and KCl we. You are given the solution of lead nitrate.
In order to obtain a yellow precipitate you should mix it with a solution of a Potassium chloride b Potassium nitride c Potassium sulphide d Potassium iodide. Option d is the answer. When a solution of lead nitrate reacts with potassium iodide it gives yellow precipitate of.
Iii lead nitrate sodium chloride Prepare separately a 5 solution of any one pair of substances listed under X and Y in water. Take a little amount of solution of Y in a conical flask and some solution of X in an ignition tube. Hang the ignition tube in the flask carefully.
See that the solutions do not get mixed. Put a cork on the flask see Fig. Thats why we have developed 5 beneficial guarantees that will make your experience with our service enjoyable easy and safe.
You have to be 100 sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Each paper is composed from scratch. To measure the solubility product of leadII sulfate PbSO 4 at 25C you construct a galvanic cell like the one shown in Figure PageIndex1 which contains a 10 M solution of a very soluble Pb 2 salt leadII acetate trihydrate in one compartment that is connected by a salt bridge to a 10 M solution of Na 2 SO 4 saturated with PbSO 4 in the other. You then insert a Pb electrode.
Suppose that small pieces of the metal nickel were placed into two separate aqueous solutions. One of iron III nitrate and one of lead II nitrate. Looking at the activity series we see that nickel is below iron but above lead.
Therefore the nickel metal will be capable of replacing the lead in a reaction but will not be capable of replacing iron. CeNi left s right cePbNO. Iii lead nitrate sodium chloride 207 g 117 g Prepare separately a 5 solution of any one pair of substances listed under X and Y each in 10 mL in water.
Take a little amount of solution of Y in a conical flask and some solution of X in an ignition tube. Hang the ignition tube in the flask carefully. See that the solutions do not get mixed.
Put a cork on the flask see Fig. Suppose we initially have a high-temperature substance such as a hot piece of metal M and a low-temperature substance such as cool water W. If we place the metal in the water heat will flow from M to W.
The temperature of M will decrease and the temperature of W will increase until the two substances have the same temperaturethat is when they reach thermal equilibrium Figure 4. When a solution of lead nitrate is added to a solution of potassium chloride for example a precipitate of lead chloride forms. We usually write the balanced reaction as a net ionic equation in which only the precipitate and those ions involved in the reaction are included.
Thus the precipitation of PbCl2 is written as Pb2aq 2Cl-aq PbCl2s In the equilibrium treatment of. The water to which you have added lead Cp 014 JC g. Solid sodium nitrate NaNO3 neutral.
The salt BX when dissolved in water produces an acidic solution. Which of the following could be true. All of the above could be true.
Which of the following is the strongest base. Kb for NH3 is 18 10-5 Ka2 for H2SO4 is 12 10-2 Ka3 for H3PO4 is 48 10-13NH3 HSO4- PO43- or. How many grams of lead II nitrate must be dissolved in 100 L of water to produce a solution that is 0300 M.
View Answer Does an excess intake of sugar and white bread increase the need for. If we mix a solution of calcium nitrate which contains Ca 2 ions with a solution of sodium carbonate which contains latextext CO_32-latex ions the slightly soluble ionic solid CaCO 3 will precipitate provided that the concentrations of Ca 2 and latextextCO_32-latex ions are such that Q is greater than K sp for the mixture. The reaction shifts to the left.
Chapter 7 shigly solution manual. Full PDF Package Download Full PDF Package. A short summary of this paper.
37 Full PDFs related to this paper. Chapter 7 shigly solution manual. Suppose we initially have a high-temperature substance such as a hot piece of metal M and a low-temperature substance such as cool water W.
If we place the metal in the water heat will flow from M to W. The temperature of M will decrease and the temperature of W will increase until the two substances have the same temperaturethat is when they reach thermal equilibrium. Question 8 predict the product for the following reaction.
Suppose for example that Hannah plans to pay off a no-interest loan from her parents. Her loan balance is 1000. She plans to pay 250 per month until her balance is 0.
The y-intercept is the initial amount of her debt or 1000. The rate of change or slope is 250 per month. We can then use slope-intercept form and the given information to develop a linear model.
Qualitative and Quantitative Analysis. We know that organic compounds are either hydrocarbons or their derivatives. Almost every organic substance contains carbon and hydrogen.
Organic molecules may also contain nitrogen sulphur halogens phosphorus and oxygen among other elements. Qualitative and quantitative analysis are two methods for detecting these elements. Since then we have taken it off line and again noticed no difference in water quality.
We will be installing a disposable salt water softener on sale at Sams club. The only good thing appears. Phosphate and nitrate cycles also need to be addressed if we are to stabilize this planet.
We are essentially awash in our own waste and global population is only beginning to flatten off. Not to mention a shrinking or flattening population brings along its own issues to solve. Probably some of the problems we will be working on in 30 years will be directly caused by near sighted solutions to.
Phosphate and nitrate cycles also need to be addressed if we are to stabilize this planet. We are essentially awash in our own waste and global population is only beginning to flatten off. Not to mention a shrinking or flattening population brings along its own issues to solve.
Probably some of the problems we will be working on in 30 years will be directly caused by near sighted solutions to. What is the theoretical yield of solid product when 255 ml of 0150 M lead II nitrate is mixed with 300 ml of 0200 M NaOH. What is the percent yield of O_2 if 102 g of O_2 is produced from the decomposition of 170 g of H_2O.
How do the actual yield and the theoretical yield from a reaction compare.