
Any gas in atmosphere that absorbs and emits radiant energy within the thermal infrared range is qualified to be consi. An excess of Al and 97 mol of Br2 are reacted according to the equation How many moles of AlBr3 will be formed assuming 100 yield.
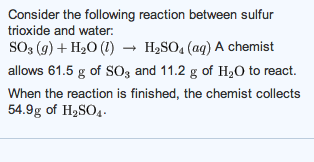
Substances Reacting Hazardously with Ammonia Acetaldehyde Magnesium Perchlorate Acrolein Mercury Boron Nitric Acid Boron Trioxide Nitrogen Tetroxide Bromine Nitrogen Trifluoride Caloric Acid Nitryl Chloride Chlorine Oxygen Difluoride Chlorine Monoxide Phosphorous Pentoxide Chlorine Trifluoride Phosphorous Trioxide Chlorites Picric Acid Chlorisilane Potassium Chromic Anhydride Potassium.
Sulfur trioxide reacting with water. Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. Clarification neededOn other planets sulfur dioxide can be found in various concentrations the most significant being the atmosphere of Venus where it is the third-most abundant atmospheric gas at 150 ppmThere it reacts with water to form clouds of sulfuric acid and is a key. Sulfur molten appears as a pale yellow crystalline solid with a faint odor of rotten eggs.
A fire and explosion risk above 450 F. Transported as a yellow to red liquid. Handled at elevated temperature typically 290F to prevent solidification and makes transfers easier.
Hot enough that plastic or rubber may melt or. COMBINATION WATER - 1837 FREE SULFUR TRIOXIDE - 0 TOTAL SULFUR TRIOXIDE - 8163. AMOUNT OF SULFUR TRIOXIDE WITH 100 SULFURIC ACID.
Ed Handbook of Chemistry and Physics. CRC Press Inc 1988-1989 p. Hazardous Substances Data Bank HSDB 4 Related Records.
41 Related Compounds with Annotation. Sulfuric acid can be obtained by dissolving sulfur trioxide in water. Physical properties Grades of sulfuric acid.
Reacting sulfuric acid with potassium nitrate can be used to produce nitric acid and a precipitate of potassium bisulfate. When combined with nitric acid sulfuric acid acts both as an acid and a dehydrating agent forming the nitronium ion NO 2 which is important. Sulfuric acid is prepared industrially by the reaction of water with sulfur trioxide.
In addition to being an oxidizing agent reacting readily at high temperatures with many metals carbon sulfur and other substances concentrated sulfuric acid is also a strong dehydrating agent combining violently with water. In this capacity it chars many organic materials such as wood paper or. Answer 1 of 7.
One word answer would be NO. But sulfur dioxide is sometimes classified as indirect greenhouse gas. The paragraph below will help you in getting better insight.
Any gas in atmosphere that absorbs and emits radiant energy within the thermal infrared range is qualified to be consi. In contact with water sulfur dioxide becomes sulfurous acid Not sulfuric acid. That requires starting with sulfur trioxide P 4 5O 2 P 4 O 10.
S 8 8O 2 8SO 2. When in contact with water the below reactions happen. A sample of river water contains 125 mg per 3dm of dissolved solids.
Calculate the mass of dissolved solids in grams in 250 cm. Of this sample of river water. Give your answer to 2 significant figures.
4 marks Mass of dissolved solids g. Do not write outside the box. A water company allows a maximum of 500 mg per dm.
Of sulfate ions in. These include controls for sulfur trioxide SO 3 hydrochloric acid HCl. Such as sodium hydroxide in water have a great affinity for SO 2.
Since such solutions are relatively expensive attempts have been made to regenerate the sodium-based scrubbing liquors by reacting or causticizing the spent scrubbing solutions with an alkaline earth compound such as lime or limestone. Reactions with Group 2 Elements. The elements of Group 2 are beryllium magnesium calcium strontium barium and radioactive radium.
Alkaline earth metals also react with oxygen though not as rapidly as Group 1 metals. These reactions also require heating. Similarly to Group 1 oxides most group 2 oxides and hydroxides are only slightly soluble in water and form basic or alkaline solutions.
Sulfur trioxide is a gas that reacts with liquid water to produce aqueous sulfuric acid or acid rain. What is the equation for this reaction. Do not worry about balancing the equation.
SO3g H2Ol H2SO4aq Aqueous magnesium chloride and hydrogen gas are produced when solid magnesium reacts with aqueous hydrochloric acid HCl. What is the equation for this reaction. Do not worry.
Phillips an English chemist patented the use of platinum to oxidize sulfur dioxide to sulfur trioxide with air. His process was employed for a time but was abandoned because of loss of activity by the platinum catalyst. Poisons in the reactants were subsequently found to be responsible and the process became a technical success at the turn of the 20th century.
In 1871 an industrial process. Sulfur trioxide in the atmosphere is naturally produced by volcanic activity but it also stems from burning fossil fuels which have traces of sulfur and from the process of roasting ores of metal sulfides in metal-refining processes. Oxides of nitrogen are formed in internal combustion engines where the high temperatures make it possible for the nitrogen and oxygen in air to.
The sulfur storage phenomena were extensively studied and a correlation was found between the catalyst sulfur storage capacity and the formation of sulfate particulates. The catalyst washcoat which stores the highest amount of sulfur is alumina. The Ptalumina catalyst is known as the most active oxidation catalyst very effective for oxidation of OF but also generating the highest sulfate.
Minute traces of such a substance can ruin the process One example is sulfur dioxide which poisons the surface of platinum and palladium. Thus all traces of sulfur compounds must be removed from the petrol used in cars fitted with catalytic converters. Further solid catalysts are much more effective if they are finely divided as this increases the surface area.
Figures 4 and 5 Two ways by. Instead it forms low melting sulfur trioxide and sodium sulfate eutectics on the surface resulting in pitting. Hot corrosion type II is a more concerning mechanism in land-based gas turbines where the presence of contaminants from lower quality fuels and operating environments such as sulfur and sodium is more frequent.
In this case the components whose lives are limited by hot corrosion. Its yellow in appearance and of course highly corrosivetoxic. HSO 3 F is generally produced by reacting hydrogen fluoride with sulfur trioxide and when combined with antimony pentafluoride it produces Magic acid a far stronger acid and protonating agent.
Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. In that case the system is at a constant pressure. Enthalpy left H right is the heat content of a system at constant pressure.
Chemists routinely measure changes in enthalpy of chemical systems as reactants are converted into products. Sulfur trioxide-Dimethylformamide complex SO3-DMF. This is a reagent required for this method of LSD production.
A completely dry 22 liter flask round bottom in an ice cooling bath is fitted with a condenser stirring device addition funnel then is filled with 10-11 liters of DMF dimethylformamide that has been freshly distilled under reduced vacuum. Use drying tubes to protect the. Calculate the molecules of oxygen required to react with 105 g of sulfur in the following reaction.
296 1023 molecules O2. For the reaction if 610 g of S is reacted with 100 g of O2 how many grams of SO3 will be produced. An excess of Al and 97 mol of Br2 are reacted according to the equation How many moles of AlBr3 will be formed assuming 100 yield.
Substances Reacting Hazardously with Ammonia Acetaldehyde Magnesium Perchlorate Acrolein Mercury Boron Nitric Acid Boron Trioxide Nitrogen Tetroxide Bromine Nitrogen Trifluoride Caloric Acid Nitryl Chloride Chlorine Oxygen Difluoride Chlorine Monoxide Phosphorous Pentoxide Chlorine Trifluoride Phosphorous Trioxide Chlorites Picric Acid Chlorisilane Potassium Chromic Anhydride Potassium. Unlike ionic hydroxides some compounds produce hydroxide ions when dissolved by chemically reacting with water molecules. In all cases.
CopperII sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid copperII oxide. The gaseous product then reacts with liquid water to produce liquid hydrogen sulfate as the only product. Write the two equations which.
Academiaedu is a platform for academics to share research papers. Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. For example in the reaction.
2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. How many moles of O₂ are required to form 500 moles of H₂O.
Please use this book to increase your knowledge for the laboratory pratictioner.