This is due to the strong hard acid Lihard base F- interaction. LiBr LiCl LiI LiF.

The results of this study show that the use of a Cd stirrer significantly improves the extent of U deposition.
Solubility of licl. Lithium chloride is a chemical compound with the formula Li ClThe salt is a typical ionic compound with certain covalent characters although the small size of the Li ion gives rise to properties not seen for other alkali metal chlorides such as extraordinary solubility in polar solvents 8305 g100 mL of water at 20 C and its hygroscopic properties. The equilibrium of a saturated LiCl aqueous solution is shown. LiCl s rightleftharpoons Li aq Cl- aq at 200 degrees C the solubility of LiCl in water is 5500 gL.
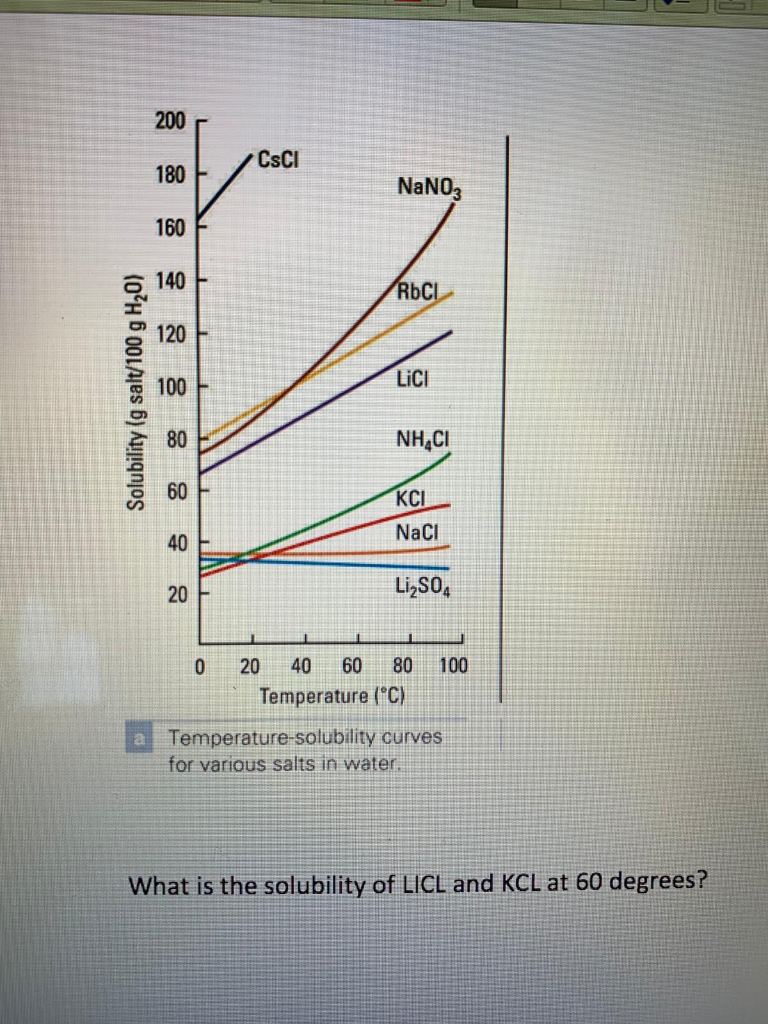
C The solubility of LiCl at 50 C is about 98 g100 g water. D The solubility of C. 12 H 22 O 11 at 50 C is about 130 g100 g water.
Determine the solubility of each of the following solid compounds at 50 C as shown in Figure 145. A NaCl b KCl c LiCl d C. 12 H 22 O 11.
Refer to the solubility behavior shown in. A new and reliable information on the solubility of salts acids and bases. Interactive and user-friendly interface.
The possibility of studying the gaming table. We use Flash technology. Soluble - soluble more than 1g per 100g of water low - low solubility 001g to 1g per 100g of water insoluble - insoluble less than 001g per 100g of water.
Based on the solubility rules will the following reaction occur because a precipitate will form. 3 Li2SO4aq 2 AlCl3aq– 6 LiCl Al2SO43 yes LiCl is insoluble yes both products are insoluble need more information no both products are soluble yes Al2SO43 is insoluble. According to the activity series for metals will the following reaction occur.
Cus ZnSO4aq. LiCl TABLE 97 Solubility Rules for Ionic Compounds in Water b. AgCl ins010BQ An ionic compound is soluble in water if it contains one of the following.
N03 C2H302 CI. Br I except when combined with Agv Pb2 or Hg22 S04 - except when combined with Ba2 Pb2 Ca2 Sr2 or Hg2 Ionic compounds that do not contain at least one. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise.
The substances are listed in alphabetical order. DNA is less soluble in isopropanol so it precipitates faster even at low concentrations. 1 The downside however is that salt will also precipitate in isopropanol.
With ethanol the DNA needs to be at a higher concentration to flocculate but the salt tends to stay soluble even at colder temperatures. DNA precipitates in 35 isopropanol and 05 M salt. At 20C the solubility of LiCl in water is 5500 gL.
A Calculate the molar concentration of LiCl. B Calculate the molar concentration of Li and Cl-. C Calculate the K sp for LiCl at 20C.
The equilibrium of a saturated NaF aqueous solution is shown below. 7 NaF s Na aq F-aq The following table shows the solubility of NaF at different temperatures. Temp C K sp.
High molecular-weight bacterial cellulose BC was found to be soluble in a lithium chlorideNN-dimethylacetamide LiClDMAc solvent system with a maximal concentration of 3 wt if an activation procedure was performed beforehand. The granular BC was not favored and had to be ground to powders before dissolution. To facilitate subsequent dissolution an activation procedure consisting.
Boiling point - the temperature at which a liquid turns into a gas. Melting point - the temperature at which a solid turns into a liquid. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. In this work the solubility of vanillic gallic syringic p-coumaric ferulic and caffeic acids was determined at 37 C under different conditions namely pure water and two different ionic media NaClaq and NaClO4aq at different ionic strengths ie 016 050 10 20 and 30 M. The solubility in water of all the acids was found to be higher than that in both of the ionic media.
Start studying Chp 13 Solubility Rules Ionic Equations Electrolyte Colloid Suspension and Solution Questions. Learn vocabulary terms and more with flashcards games and other study tools. Copyright 2021 Claude Yoder.
The following table summarizes the results of testing in boiling salt solutions of 26 NaCl sodium chloride 33 LiCl lithium chloride and 42 MgCl2 magnesium chloride. The boiling LiCl and MgCl2 test solutions are very aggressive relative to practical applications and only austenitic alloys with compositions that approach those of nickel-base alloys will routinely resist cracking in. Aqueous Solubility Silver Halides Compound solubility product AgF 205.
AgCl 18 x 10-10. AgBr 52 x 10-13. AgI 83 x 10-17.
Ol Ag aq X-aq Solubility of Lithium Halides. LiBr LiCl LiI LiF. LiF should have a higher solv than the other salts yet it is the least soluble in water.
This is due to the strong hard acid Lihard base F- interaction. Three additional runs were conducted using LiCl-KCl-UCl 3-RECl 3 to determine the extent of URE electrodeposition. The maximum number of moles of U RE metals deposited was 007 a value estimated to be 214 times higher than the solubility limits exhibited by these metals in Cd.
The results of this study show that the use of a Cd stirrer significantly improves the extent of U deposition. Solubility in water is related to the ionic nature and size. Smaller ions have higher charge density and can be solvated by more water molecules.
This releases a higher enthalpy of hydration and makes the hydrated ions more stable. Solubility of Be 2 Solubility of Mg 2 Solubility of Ca 2 Solubility of Sr 2 Solubility of Ba 2 Reactivity of Alkaline Earth Metals. The s-block is one of four blocks of elements in the periodic tableThe element of s- group have a common propertyThe electron in their most outward electron shell are in the s-orbital.
Elements in the s- are in the first two periodic table groups. The elements in group one are called the alkali metalsThe elements in group two are called the alkaline earth metals. Lithium carbonate Li 2 CO 3 exhibits the remarkable property of retrograde solubility.
It is less soluble in hot water than in cold. Lithium and its compounds impart a crimson colour to a flame which is the basis of a test for its presence. It is commonly kept in mineral oil because it reacts with the moisture in the air.
Organolithium compounds in which the lithium atom is not present as. Size effect in ionic bond adds to the lattice energy of ionic solids and hence determines their solubility in water. Image Will be Uploaded Soon The example above shows that the sodium atom gives out its one valence electron to the atom of chlorine.
This leads to the formation of one sodium and one chlorine cation and anion respectively. Note that the resulting compounds charge is. Neodymium Fluoride NdF 3.
Good Tungsten Molybdenum Mo W-Mo Ta Al 2 O 3 RF. Neodymium Oxide Nd 2 O 3 1900-724—1400. Good Tantalum Tungsten Ta W– ThO 2 RF RF-R.