Temperature affects the solubility of both solids and gases but hasnt been found to have a defined impact on the solubility of liquids. Discover what precipitates are and learn about the solubility rules net.
Helium has many unique properties.
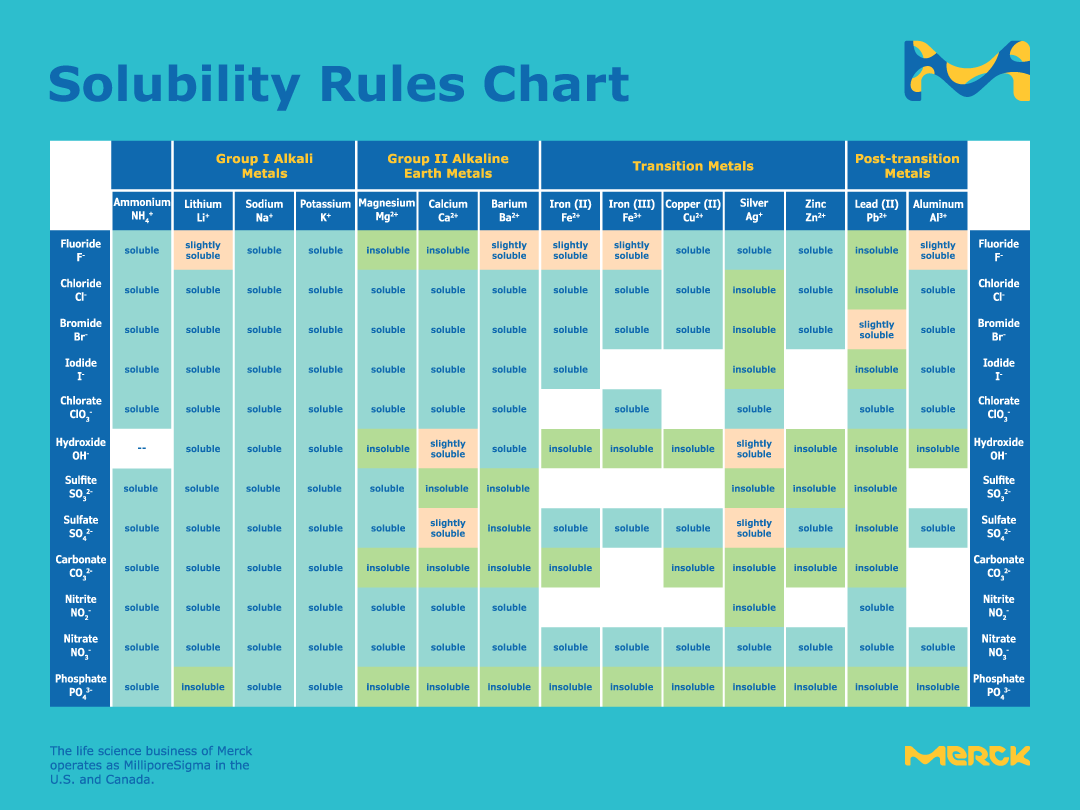
Solubility chart with mercury. A solubility chart is a chart with a list of ions and how when mixed with other ions they can become precipitates or remain aqueous. The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx. 25 C 29815 K.
Any box that reads soluble results in an aqueous product in which no precipitate has. One of the most insoluble ionic compounds is mercury II sulfide. Oxalates are highly insoluble except for Na K and NH 4.
Even rubidium and cesium oxalate are insoluble. Solubility Chart Solubility Chart of common anions and cations. This solubility chart shows the solubility of common ions.
A white space means that the compound is not stable in aqueous solution. Chart is provided by. Ksp solubility product constants of many popular salts at SolubilityOFthings.
Hg 2 Br 2. Hg 2 CO 3. Hg 2 Cl 2.
Hg 2 F 2. Hg 2 I 2. Hg 2 C 2 O 4.
Hg 2 SO 4. 65 x 10-7. Compounds of Group 1 elements Li Na K Rb Cs and Fr or ammonium NH 4 are soluble.
Nitrates NO 3 chlorates ClO 3 perchlorates ClO 4 and acetates C 2 H 3 O 2 are soluble. Chlorides Cl bromides Br and iodides I are soluble except for those of Ag Pb 2 and Hg 2 2. With the exception of rule 1.
AgOAc silver acetate and HgOAc 2 mercury acetate are insoluble. AgNO 2-and KClO 4-are only slightly soluble 6. Note that compounds of Cl- Br- and I-are usually soluble.
The chloride bromide and iodide ions almost always make soluble compounds called halogen salts. If any of these pair with the ions silver Ag mercury Hg 2 2 or lead Pb 2 the result. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise.
The substances are listed in alphabetical order. Temperature affects the solubility of both solids and gases but hasnt been found to have a defined impact on the solubility of liquids. Pressure can also affect solubility but only for gases that are in liquids.
Henrys law states that the solubility of a gas is directly proportional to the partial pressure of the gas. Solubility and Related Thermodynamic Quantities of CadmiumII Carbonate in Aqueous Systems JPCRD 2011 40 043104. Jitka Eysseltováa and Roger Bouaziz Potassium Sulfate in Water JPCRD 2012 41 013103.
Liquids bromine mercury and water All other elements are solids. When in doubt so are most other compounds particularly ionic compounds. Composition and Decomposition Reactions Must Use.
Common Sense Tips Ionic Compounds are solids Molecular Compounds either liquid or gas Diatomics usually gas. Examples S s H 2g H 2 S g Cu s S s CuS s O 2g H. Furthermore the solubility of this species increases with temperature.
We add 1-2 mL of deionized H 2 O and put the test tube in bain-marie by heating not boiling and shaking the solution with the glass rod. We quickly bring the still hot solution in a. Barium is a silvery-white metal that can be found in the environment where it exists naturally.
It occurs combined with other chemicals such as sulfur carbon or. Magnetic material requires special sputter source One run only Influenced by composition The z-ratio is unknown. Therefore we recommend using 100 or an experimentally determined value.
Please click here for instructions on how to determine this value. All metals alumina coated. Incl Inconel.
__ and mercury are heavy metals that are still used in germicidal preparations. Which of following compounds are commonly used as gaseous sterilants or disinfectants. Ethylene oxide chlorine dioxide.
Heavy metal are reliably. Surgical handscrubbing is. Helium has many unique properties.
Low boiling point low density low solubility high thermal conductivity and inertness so it is use for any application which can explioit these properties. Helium was the first gas used for filling balloons and dirigibles. This application goes on in altitude research and for meteorological balloons.
The main use of helium is as an inert protection gas in. The City of Salisbury NC is the county seat of Rowan County North Carolina United States. The population was 33663 in the 2010 Census growing 278 from the previous Census in 2000.
Salisbury is the home to famed North Carolina soft drink Cheerwine and regional supermarket Food Lion. It is one of only two cities in North Carolina to have Internet up to 10 gigabits per second through its. A precipitation reaction is a process during which two reactants form a precipitate.
Discover what precipitates are and learn about the solubility rules net. Separated from other similar ions and then tested for. The separations are based on solubility differences.
The ions we will be testing for are Ag Pb2 Hg 2 2. This lab will consist of two parts. In Part I you will follow the flow chart on the next page with ten drops of a known solution which contains all three ions.
In Part II you will. Moderate heat resistance to 130 C or 270 F with good gloss and transparency. Series has excellent re-solubility for minimal press problems clean wiping with very good transfer properties that result in trouble free defect free printing.
Anhydrous ammonia NH 3. A colorless non-flammable liquefied gas. Vapor is lighter than air - 06 compared to air 10 ignition temperature 1204 o F 651 o Cvapor concentration between 15 and 28.
Corrodes galvanized metals cast iron copper brass or copper alloys. The solubility equation developed by Yalkowsky can be used to estimate intrinsic water. It provides a chart with some guidance on categorization of different physical hazards based on common labeling standards.
EPAs DfE evaluates physical hazards using the United Nations GHS which is an internationally recognized structure for communication of a range of hazards UNECE 2013a. Solubility Chart for Inorganic Salts. Reduction and Oxidation Potentials for Ion Radicals.
Enthalpy of Hydration of Gases. Thermophysical Properties of Water and Steam. Vapor Pressure and Other Saturation Properties of Water.
Standard Density of Water. Fixed-Point Properties of H₂O and D₂O. Properties of Saturated Liquid D₂O.
Properties of Ice and Supercooled Water. Get personalized online tutoring from expert tutors at TutorEye. We are the leading platform to provide one on one tutoring sessions to K-12 college students.
Hire best homework helpers for online homework help 247. Inorganic Compounds in Water - Melting and Boiling Temperatures Densities and Solubility - Physical constants for more than 280 common inorganic compounds. Density is given for the actual state at 25C and for liquid phase at melting point temperature.
You have remained in right site to begin getting this info. Jalandhar India 2012. The reagent group mercury triflouro acetatewatersodium borohydride codes for Dehydrating agent used to dehydrate alcohols to alkenes.
Feb 3 2021 Master Organic Chemistry Reagent Guide Pdf Free 11. You can get most of books or related to your search from websites like z library.