However the resulting ECF loss will stimulate the renin-angiotensin-aldosterone system as a means to increase sodium and hence water retention Share et al 1972. Powder for oral solution.

Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47.
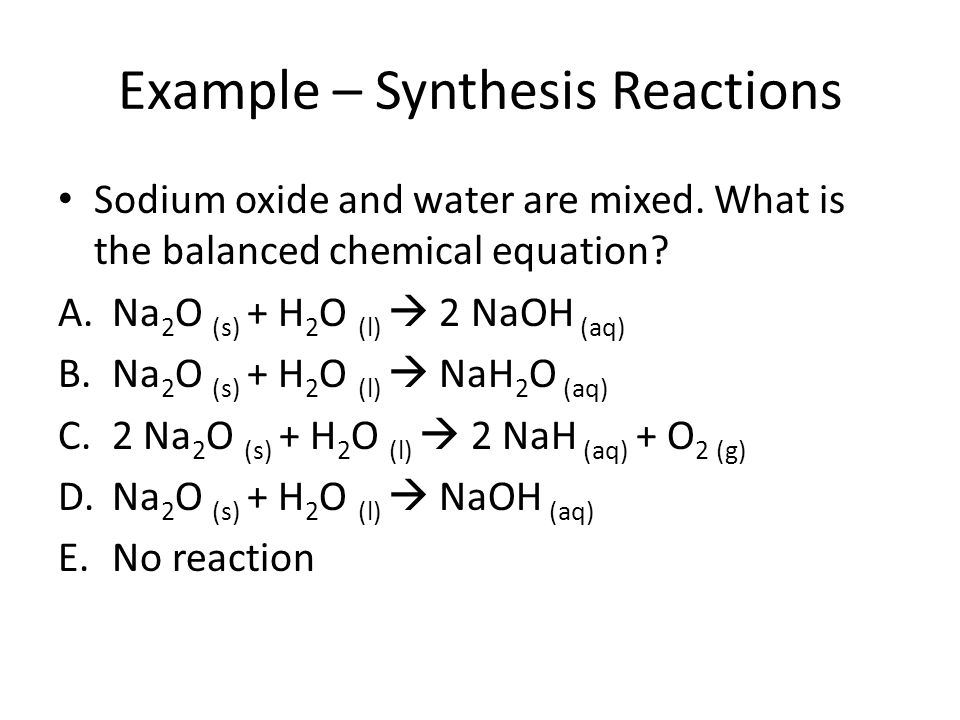
Sodium oxide and water reaction. Sodium oxide is a chemical compound with the formula Na 2 O. It is used in ceramics and glasses. The compound is the base anhydride of sodium hydroxide.
When water is added to sodium oxide NaOH is produced. Na 2 O H 2 O 2 NaOH. The alkali metal oxides M 2 O M Li Na K Rb crystallise in the antifluorite structure.
In this motif the positions of the anions and cations are reversed. Sodium oxide reacts with carbon dioxide to form sodium carbonate. The chemical equation is given below.
2Na 2 O 3CO 2 2Na 2 CO 3. Sodium oxide reacts with some acids such as hydrochloric acid to form sodium chloride and water. The chemical equation for this reaction is given below.
Na2O 2HCl 2NaCl H2O. Uses of Sodium Oxide. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. Answer 1 of 16.
Sodium metal reacts rapidly with water to form a colourless solution of sodium hydroxide NaOH and evolve hydrogen gas H2. The resulting solution is basic because of the dissolved hydroxide. The reaction is exothermic.
During the reaction the sodium metal may well become so. Water appears as a clear nontoxic liquid composed of hydrogen and oxygen essential for life and the most widely used solvent. Include water in a mixture to learn how it could react with other chemicals in the mixture.
Water is an oxygen. Layer of aluminum oxide thus allowing the reaction with water to proceed. The hydrogen produced via such aluminum-water reactions might be employed to power fuel cell devices for portable applications such as emergency generators and laptop computers.
There is also the suggestion that aluminum-water reactions might be used for hydrogen storage on fuel cell-powered vehicles. The purpose of. The reaction of sodium with alcohols is similar to the reaction of sodium with water but slower.
There are two general reactions with organic halides. One of them requires the condensation of two organic compounds which form halogens when those are eliminated. The second type of reaction includes the replacement of halogen by sodium to obtain a sodium organic compound.
Oxidation of metals Sodium hypochlorite reacts with metals and forms metal oxide by oxidizing them. Reaction is given below NaOCl Mg MgO NaCl. Reaction with ammonia - Sodium hypochlorite reacts with NH 3 and forms sodium hydroxide and NH 2 Cl.
Sodium picosulfate 100mg. Magnesium oxide light 35g. Citric acid anhydrous 120g - Each sachet also contains.
Potassium hydrogen carbonate 05g equivalent to 5 mmol 195 mg potassium Lactose as a component of the flavour For the full list of excipients see section 61. Powder for oral solution. Place about 125 cm 3 of water in a 250 cm 3 conical flask.
Add one or two drops of phenol red to the water. Add two drops of sodium hydroxide solution to produce a red solution. Talk or blow gently into the flask ie add carbon dioxide.
Continue adding the carbon dioxide until a colour change is observed. Questions for the class. Carbon dioxide and water will be given off leaving dry sodium carbonate.
This is the soda ash. The chemical reaction for the process is. 2 NaHCO 3 s Na 2 CO 3 s CO 2 g H 2 Og The compound will readily absorb water forming the hydrate returning to baking soda.
You can store the dry sodium carbonate in a sealed container or with a desiccant to keep it dry or you can allow it. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and. In a randomized multicentre rater-blinded study in adults bowel cleansing prior to colonoscopy using two different regimens of CitraFleet was compared with that following Klean-Prep each sachet containing 59 g polyethylene glycol 3350 5685 g anhydrous sodium sulfate 1685 g sodium bicarbonate 1465 g sodium chloride and 07425 g potassium chloride.
To be dissolved in 1. Sulfuric acid barium hydroxide — barium sulfate and water. Silver nitrate sodium chloride — silver chloride and sodium nitrate.
You can also cause a double replacement chemical reaction when you combine an acid and a base. Reactions that use an acid and a base as reactants is known as a neutralization reaction. They dont cancel.
Neutralisation is a reaction between an acid and an alkali that forms a salt and water. Salts are odourless and have a salty taste and many are soluble in water. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate.
The pH scale is used to measure acidity and alkalinity. Indicators are substances that change colour with a change in acidityalkalinity. The color is created by the reaction of nitric oxide formed from sodium nitrite with myoglobin to form nitric oxide myoglobin.
Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002. Pehrsson et al 2008. Tea and coffee are also very low in sodium although the level may increase slightly with the.
When sodium is cut or scratched its freshly exposed shiny surface rapidly turns dull as a thin layer of sodium oxide forms. Sodium oxygen sodium oxide 4Nas O 2 g 2Na 2 Os. Sodium AcetateCH3COONa- Sodium acetate is the salt of acetic acid and sodium hydroxide.
It is widely used across a number of industrial sectors. It is hygroscopic in nature and easily soluble in water. It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid.
To learn more about Sodium Acetate Preparation Properties Uses and FAQs Visit BYJUS for a. Reacts very vigorously with gaseous hydrogen sulfide. Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47.
An explosion results when gaseous carbon dioxide is passed over a mixture of sodium peroxide with powdered magnesium Mellor 2490 1946-47. When solute and water are lost proportionately such as with diarrhea or vomiting osmolality remains constant and vasopressin release is blunted. However the resulting ECF loss will stimulate the renin-angiotensin-aldosterone system as a means to increase sodium and hence water retention Share et al 1972.
This mechanism appears to be less. You also have a lot of sodium in the water and will need an RO System. RO is really the only way to reduce sodium levels that high.
If you are only concerned about drinking water then you could do an under the counter RO unit for drinking water at the kitchen sink. Or to have drinkable water throughout the house you will need a whole-house RO system. November 15 2015 1259 am.
2360 moles of lead II oxide. 0031 moles of aluminium iodide. 1077 moles of magnesium phosphate.
050 moles of calcium nitrate. Convert the following masses into their corresponding number of moles. 235 g of sodium chloride.
0778 g of sodium cyanide. 0250 g of water. 16945 g of calcium acetate.
799 g of potassium permanganate. Moles Number of. Mechanism For The Reduction Of Aldehydes And Ketones With NaBH 4.
The mechanism of the reaction of sodium borohydride with aldehydes and ketones proceeds in two stepsIn the first step H detaches from the BH 4 and adds to the carbonyl carbon an example of 12-addition. This forms the C-H bond and breaks the C-O bond resulting in a new lone pair on the oxygen. Metallic sodium and chlorine gas are produced by the electrolysis of molten sodium chloride.
Electrolysis of an aqueous solution of sodium chloride yields sodium hydroxide and chlorine gas. Hydrogen and oxygen are produced by the electrolysis of water. Types of Chemical Reactions.
Can you identify which type of chemical reaction is shown. Test your knowledge with this quiz. Access the most up to date content in ISO standards graphical symbols codes or terms and definitions.
Preview content before you buy search within documents and easily navigate between standards.