Cool filter and collect the filtrate in a test tube and label it as sodium. Some boron compounds such as boron nitrite are completely water insoluble.
Sodium azide is the inorganic compound with the formula NaN 3This colorless salt is the gas-forming component in legacy citation needed car airbag systems.
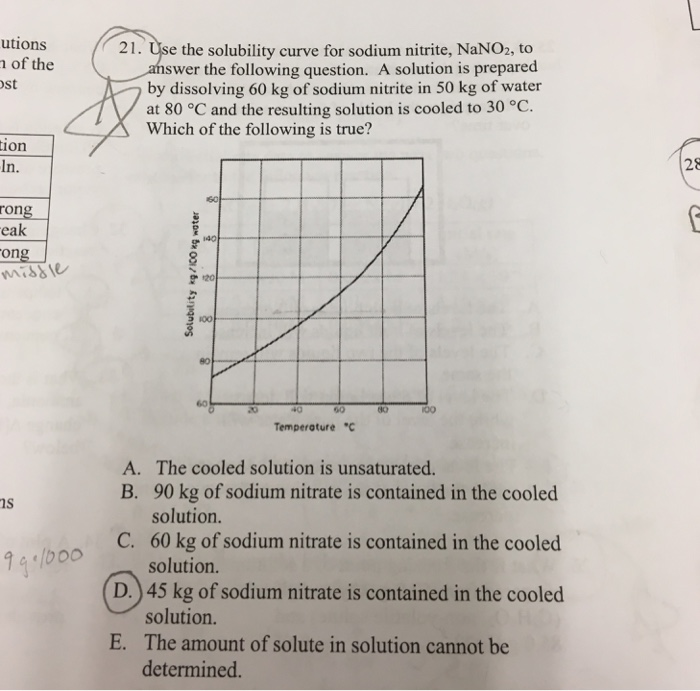
Sodium nitrite solubility. Sodium nitrite is sold as the salt and in solution. The finely crystalline slightly yellowish salt is marketed in untreated form and also after treatment with aryl alkyl sulfonates. The salt contains ca.
990 sodium nitrite 06 sodium nitrate 01 sodium chloride and sodium sulfate and 01 water. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. Sodium nitrate is a white deliquescent solid very soluble in water.
Sodium azide is the inorganic compound with the formula NaN 3This colorless salt is the gas-forming component in legacy citation needed car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance is highly soluble in water and is very acutely poisonous.
The taste threshold for sodium in water depends on the associated anion and the temperature of the solution. At room temperature the threshold values are about 20 mglitre for sodium carbonate 150 mglitre for sodium chloride 190 mglitre for sodium nitrate 220 mglitre for sodium sulfate and 420 mglitre for sodium bicarbonate 6. Freely soluble in water very slightly soluble in ethanol.
55 to 80 10 aqueous solution Whatre the Application of Sodium Erythorbate. The cured meats in the human diet have a history of thousands of years. Sodium erythorbate is used primarily to bring out a nice red color in sausage ham bacon and other cured meat products or fish products as it.
The solubility of NaNO 3 in water corresponds to 912g100mL at a temperature of 25 o. This compound is also highly soluble in ammonia. When dissolved in water sodium nitrate dissociates into Na and NO 3 It is a very strong oxidizing agent.
It reacts violently with reducing agents. At high temperatures this compound is known to explosively decompose. To easily determine solubility memorize the most popular soluble chemicals and compounds.
Remember that elements such as lithium sodium potassium or other alkali metals as well as chloride bromide and iodide are all soluble. Additionally any compounds that contain nitrate acetate nitrite chlorate or perchlorate are soluble. This Sodium bisulfite releases sulfur dioxide gas which kills yeast and other bacteria in wines.
It is also used in pipelines and other metallic surface made of iron to prevent corrosion. Cyanide poisoning is treated by using antidote with a combination of vitamin-B12 sodium nitrite and sodium thiosulfate. Adult Until sodium nitrite becomes available break one ampule of amyl nitrite into a cloth.
Out of every minute hold the cloth containing amyl nitrite in front of the patients mouth for 30 seconds and then remove it for 30 seconds until sodium nitrite can be administered. A new ampule of amyl nitrite should be broken into a cloth every 3 minutes. Discontinue use of amyl nitrite when.
Solubility Rules or Table 104 on page 231 of your textbook. LeadII acetate potassium hydroxide PbOH2 sodium chromate nickelII iodide NiCrO4 strontium perchlorate ironII sulfate SrSO4 ammonium acetate cadmium nitrate none thalliumI nitrate calcium chloride TlCl sodium sulfide ironIIIchloride Fe2S3 NiCrO4 SrSO4 none PbOH2 TlCl Fe2S3 13035035 points Previous. For more Solubility Complete data for POTASSIUM NITRITE 6 total please visit the HSDB record page.
Hazardous Substances Data Bank HSDB Solubility in water g100ml at 0 C. 281 very good ILO International Chemical Safety Cards ICSC 326 Density. The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals.
Sodium thiosulfate commonly known as sodium thiosulphate is a medicine that is used to treat cyanide poisoning pityriasis versicolor and cisplatin side effects. It is frequently used after the drug sodium nitrite for cyanide poisoning and is usually only prescribed in severe situations. Sodium Thiosulfate is a toxin with a modest toxicity.
Sodium carbonate extract is given below. Preparation of sodium carbonate extract Take 1g of salt in a porcelain dish or boiling tube. Mix about 3 g of solid sodium carbonate and add 15 mL of distilled water to it.
Stir and boil the content for about 10 minutes. Cool filter and collect the filtrate in a test tube and label it as sodium. Boiling point - the temperature at which a liquid turns into a gas.
Melting point - the temperature at which a solid turns into a liquid. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
For optimum solubility we recommend mixing with 1 L 1 qt of water the resulting mixture will have a white opaque appearance. When this mixture is added to the aquarium it will impart a slight haze that should clear within 1530 minutes. Do not use Equilibrium when replacing evaporated water.
This dose is based on DI or RO water. For other water measure hardness GH first. Salt Solubility in cold water glitre Solubility in hot water glitre Sodium chloride 357 391 Potassium chloride 344 567 Calcium chloride 745 1590 Organoleptic properties The taste threshold of the chloride anion in water is dependent on the associated cation.
Taste thresholds for sodium chloride and calcium chloride in water are in the. Na 3 PO 4. NaKC 4 H 4 O 6.
Na 2 SO 4. Na 2 SO 3. Na 2 C 4 H 4 O 6.
0-28 20C 159. The most widely applied nitrogen fertilizers are probably NaNO 3 sodium nitrate and NH 4 NO 3 ammonium nitrate. After fertilization crops take up a relatively small part of added nitrogen compounds namely 25-30.
The residue ends up in groundwater and surface water through soils because nitrates are water soluble. Organic fertilizers mainly contain nitrogen as proteins urea or. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
This is our newest publication and has been created to support the school technician profession in Scotland. A Reaction with nitrous acid Dissolve the amine 05 mL in concentrated acid 20 mL and water 3 mL and cool the solution to 0 - 5 in an ice-bath for 5 minutes. Add a cold solution ice-bath of sodium nitrite 05 g in water 20 mL from a dropper with swirling of.
Solubility of boron and boron compounds. Boron salts are generally well water soluble. Boric acid has a water solubility of 57 gL borax of 252 gL and boron trioxide of 22 gL.
Boron trifluoride is the least water soluble boron compound with a water solubility of 24 gL. Some boron compounds such as boron nitrite are completely water insoluble. Why is boron present in water.
If the anion is a polyatomic ion its suffix can vary but is typically either ate or iteas in the cases of sodium phosphate and calcium nitrite depending on the identity of the ion. Li and F combine to form LiF. Ca 2 and Cl combine to form CaCl 2.
Fe 2 and. Examples include sodium nitrate seaweed bones guano potash and phosphate rock. Compounds can also be chemically synthesized from basic raw materials.
These would include such things as ammonia urea nitric acid and ammonium phosphate. Since these compounds exist in a number of physical states fertilizers can be sold as solids liquids or slurries. This website uses cookies to help provide you with the best possible online experience.
Please read our Terms Conditions and Privacy Policy for information about. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában.