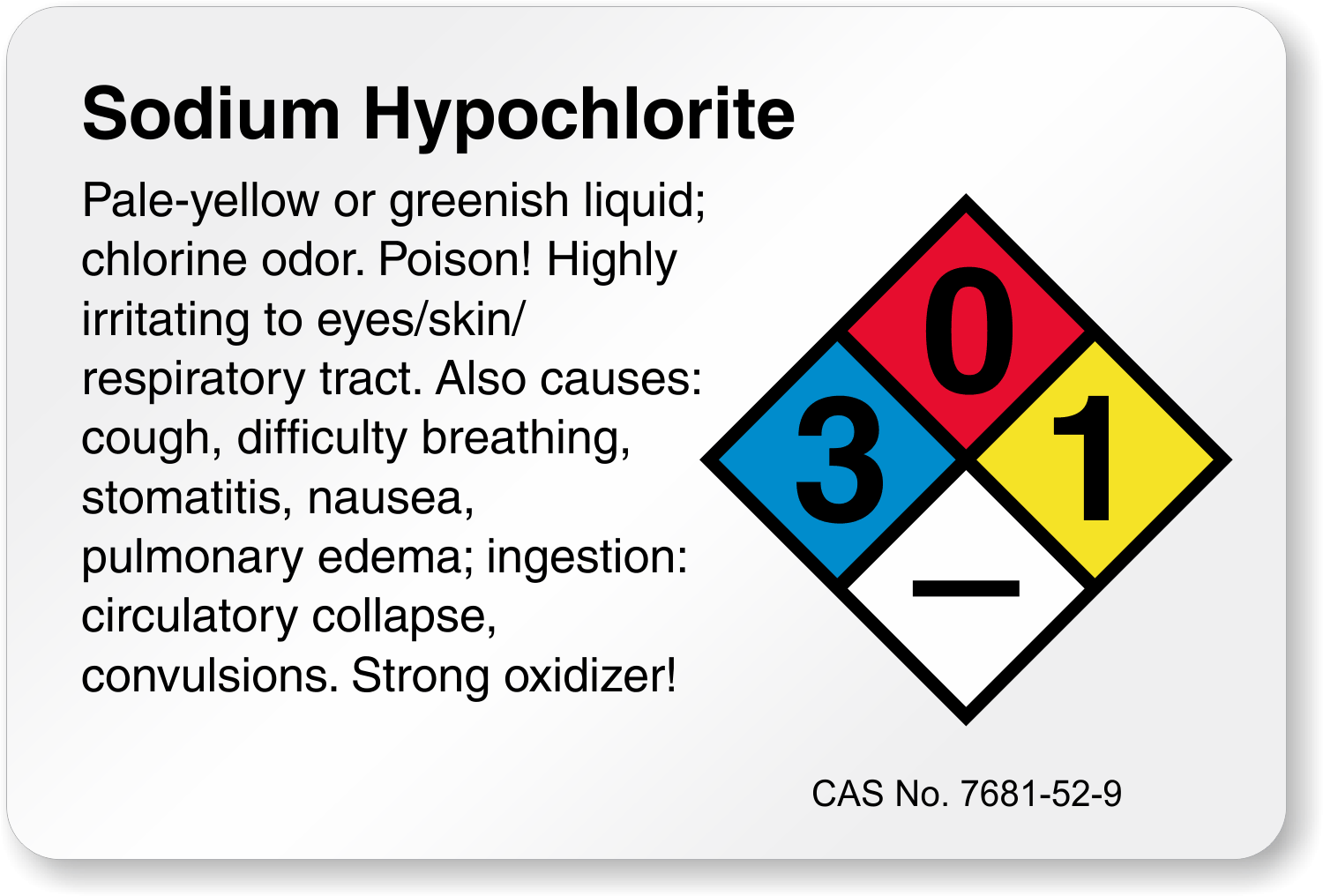
Sodium Hypochlorite is a clear slightly yellow or green liquid with a strong Chlorine odor. Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO.

Safety hazards of Calcium Hypochlorite to potentially exposed workers.
Sodium hypochlorite hazards. Soln of sodium hypochlorite containing 045-050 g of the salt in 100 ml. Prepd by diluting with distilled water a soln of sodium hypochlorite adding a 5 soln of sodium bicarbonate nad adjusting to proper strength and concn according to procedure described in NF. May be prepared also from 154 g chlorinated lime 30 available chlorine 77 g anhydrous sodium carbonate and 64 g sodium.
Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively.
It can be crystallized as a pentahydrate NaOCl 5 H 2 O a. Sodium hypochlorite is the chemical compound known largely as bleach when dissolved in water. Pure anhydrous without water sodium hypochlorite is very unstable and subject to intense chemical even explosive reactions in the presence of heat friction or other chemicals and is not commonly utilized.
A solution of sodium hypochlorite is a clear yellow to green liquid with an immediately. Special Remarks on Explosion Hazards. Anydrous Sodium Hypochlorite is very explosive.
Primary amines and calcium hypochlorite or sodium hypochlorite react to form normal chloroamines which are explosive. Interaction of ethyleneimine with sodium or other hypochlorite gives the explosive N-chloro cmpd. Removal of formic acid from industrial waste streams with sodium hypochlorite soln becomes.
Exposure Hazards Routes of Exposure. Inhalation ingestion skin contact eye contact. Sodium hypochlorite has a pronounced irritant effect and may cause severe burns to skin and eyes.
Poisonous vapor chlorine gas is corrosive to respiratory passages and may cause irritation of mouth nose and throat. If ingested sodium hypochlorite is poisonous causes burns abdominal cramps nausea. 125 SODIUM HYPOCHLORITE SOLUTION Material Safety Data Sheet MSDS No.
060103 Page 2 of 4 HEALTH HAZARDS Signs and Symptoms of Exposure. Eyes and skin irritation. Chemical burns to broken skin.
Medical Conditions Aggravated by Exposure. Oral ingestion LD 50. Dermal skin absorption LD 50.
Sodium Hypochlorite SPECIFIC HAZARDS Chlorine. PROTECTIVE MEASURES IN FIRE Self contained breathing apparatus and full protective clothing must be worn in case of fire. 6 ACCIDENTAL RELEASE MEASURES PERSONAL PRECAUTIONS Wear protective clothing as described in Section 8 of this safety data sheet.
ENVIRONMENTAL PRECAUTIONS Do not allow ANY environmental. The Hazard fields include special hazard alerts air and water reactions fire hazards health hazards a reactivity profile and. Sodium hypochlorite 15 7681-52-9.
Liquid 480 480 480 480 480 480 480 480 480 480. Sodium hypochlorite 30 7681-52-9. Liquid 480 480 480.
Sodium hypochlorite 6 7681-52-9. Liquid 480 indicates greater than. A blank cell indicates the fabric.
Bleach is the generic name for any chemical product that is used industrially and domestically to remove color from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically to a dilute solution of sodium hypochlorite also called liquid bleach. Many bleaches have broad spectrum bactericidal properties making them useful for.
Caustic soda Lye Sodium hydroxide Soda lye Sodium hydrate CAS No. DOT ID Guide. 1823 154dry solid 1824 154solution.
Sodium Hypochlorite is a clear slightly yellow or green liquid with a strong Chlorine odor. It is usually mixed in water and used as a household cleaner a disinfectant and a bleaching agent. It is also used in water treatment and purification.
Reasons for Citation f Sodium Hypochlorite is on the Right to Know Hazardous Substance List because it is cited by DOT IARC and EPA. F This chemical. Product Name Sodium hypochlorite Cat No.
AC219255000 CAS-No 7681-52-9 Synonyms Antiformin Recommended Use Laboratory chemicals. Uses advised against Food drug pesticide or biocidal product use. Details of the supplier of the safety data sheet Emergency Telephone Number For information US call.
001-800-ACROS-01 Europe call. Hypochlorite is an ion composed of chlorine and oxygen with the chemical formula ClO. Being unstable in the pure form hypochlorite is most commonly used for bleaching disinfectation and water treatment purposes in its salt form sodium hypochloriteHypochlorite is often used as a chemical reagent for chlorination and oxidation reactions.
Sodium hypochlorite 7681-52-9 5 - 10 The exact percentage concentration of composition has been withheld as a trade secret. FIRST AID MEASURES First aid measures General Advice Call a poison control center or doctor immediately for treatment advice. Sodium hypochlorite 7681-52-9 100 - 500 Sodium chloride 7647-14-5 100 - 500 Sodium hydroxide 1310-73-2 100 - 500 Sodium silicate 1344-09-8 100 - 500 The specific chemical identity andor exact percentage concentration of this composition has been withheld as a trade secret.
Safety Data Sheet. Safety hazards of Calcium Hypochlorite to potentially exposed workers. CALCIUM HYPOCHLORITE page 2 of 6 This Fact Sheet is a summary source of information of all potential and most severe health hazards that may result from exposure.
Duration of exposure concentration of the substance and other factors will affect your susceptibility to any of the potential effects described below. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. This is our newest publication and has been created to support the school technician profession in Scotland.
Oxidizing materials are liquids or solids that readily give off oxygen or other oxidizing substances such as bromine chlorine or fluorine. They also include materials that react chemically to oxidize combustible burnable materials. This means that oxygen combines chemically with the other material in a way that increases the chance of a fire or explosion.
For example the toxic gas chlorine can be released if you mix sodium hypochlorite bleaching solutions and acidic cleaning agents. Mixing a disinfectant with anything else could change its properties and it may no longer be effective. Health hazards and protective measures for chemicals used as disinfectants.
The table below provides information about health hazards associated with. Products Potential Hazards Controls. Alcohols Ethyl alcohol o Clorox 4 in One Disinfecting Spray Ready-to-Use Isopropyl alcohol o Isopropyl Alcohol Antiseptic 75 Topical Solution MM Ready to Use o Opti-Cide Surface Wipes o Powell PII Disinfectant Wipes o Super Sani Cloth Germicidal Wipe Highly flammable and could form explosive vaporair mixtures.
May react violently with strong oxidants. Exposure to a dilute 1-10 sodium hypochlorite or calcium hypochlorite solution. MS222 or rapid chilling as described above.
Exposure to a dilute sodium hypochlorite or calcium hypochlorite solution. Methods of Confirmation of Euthanasia. Corrosivity or hazards associated with the chemicals in the disinfectant.
70 Ethanol Aqueous alcohol solutions are not appropriate for surface decontamination because of the evaporative nature of the solution. A contact time of ten minutes or more is necessary and not achievable using a 70 vv aqueous solution of ethanol. 70 ethanol can be used to soak small pieces of.
Specific Hazards Arising from the Chemical. This product causes burns to eyes skin and mucous membranes. Thermal decomposition can release sodium chlorate and irritating gases and vapors.
Explosion Data Sensitivity to Mechanical Impact. Sensitivity to Static Discharge. Protective equipment and precautions for firefighters.
As in any fire wear self-contained breathing. Chlorine bleach is sometimes called sodium hypochlorite or hypochlorite You will encounter it in chlorine bleach automatic dishwashing detergents chlorinated disinfectants and cleaners chlorinated scouring powder mildew removers and toilet bowl cleaners. Do not mix products together.
Do not mix them with ammonia or vinegar. Read the labels of products in your home and. The specific chemicals in these ingredients include ammonia ethylene glycol monobutyl acetate sodium hypochlorite andor trisodium phosphate.
Depending on the ingredients used all-purpose cleaners can irritate the skin eyes nose and throat. They can be highly poisonous to both humans and animals if swallowed. When working with an all-purpose cleaner always wear rubber gloves.