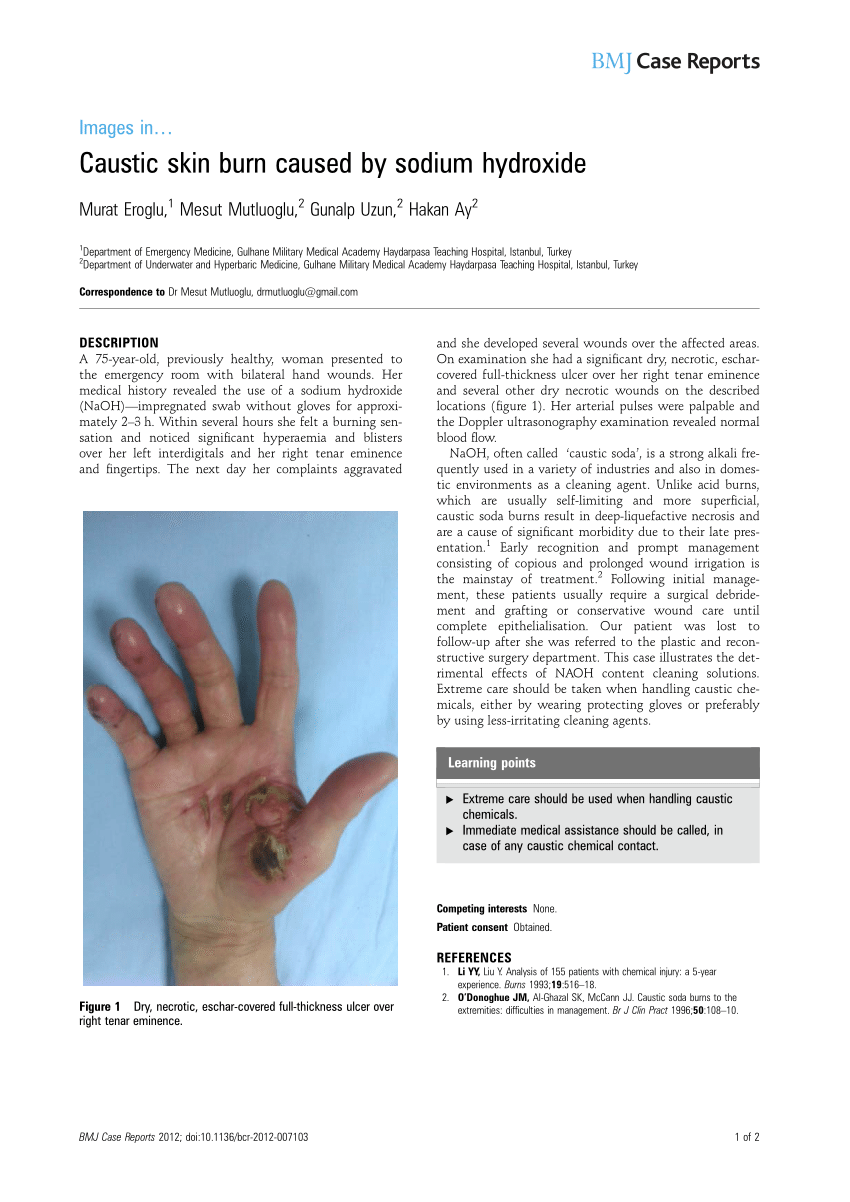
Inspect the glassware prior to use to make sure it is free from. Dilute sodium or potassium hydroxide solution Sodium hydroxide If less than 05 M but 0125 M or more.

A severe exposure can cause death.
Sodium hydroxide burns. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
Contact can cause pain redness burns and blistering. Permanent scarring can result. A severe exposure can cause death.
Burns may not be immediately painful. Onset of pain may be delayed minutes to hours. Contact causes severe burns with redness swelling pain and blurred vision.
Permanent damage including blindness. Solid sodium hydroxide or solutions containing high concentrations of sodium hydroxide may cause chemical burns permanent injury or scarring and blindness. Solvation of sodium hydroxide is highly exothermic and the resulting heat may cause heat burns or ignite flamables.
The combination of aluminium and sodium hydroxide results in a large production of hydrogen gas. 2Als 6NaOHaq. Sodium hydroxide NaOH is a an odorless white crystalline solid that absorbs moisture from the air.
Sodium hydroxide is very corrosive. It can cause irritation to the eyes skin and mucous membrane. Eye and skin burns.
And temporary loss of hair. Workers may be harmed from exposure to sodium hydroxide. The level of harm depends upon the dose duration and work being done.
Sodium hydroxide is a powerful and extremely corrosive alkali it decomposes living tissues. Eye contact of NaOH can cause permanent blindness. Skin contact with NaOH is a reason for severe chemical burns.
Sodium hydroxide solvation is highly exothermic can cause splashing to burn. It is caustic and could cause chemical burns. Into a large volume of water and then dilute the solution to make one liter.
Add sodium hydroxide to water do not add water to solid sodium hydroxide. Be sure to use borosilicate glass eg Pyrex and consider immersing the container in a bucket of ice to keep the heat down. Inspect the glassware prior to use to make sure it is free from.
Skin contact with sodium hydroxide can cause severe burns with deep ulcerations. Pain and irritation are evident within 3 minutes but contact with dilute solutions may not cause symptoms for several hours. Contact with the eye may produce pain and irritation and in severe cases clouding of the eye and blindness.
Long-term exposure to sodium hydroxide in the air may lead to ulceration of the. Causes severe skin burns and eye damage. Potassium hydroxide is also harmful if swallowed if 3 M or more.
Fehlings solution contains sodium hydroxide of this concentration. It is used in the home as an oven cleaner. Dilute sodium or potassium hydroxide solution Sodium hydroxide If less than 05 M but 0125 M or more.
Potassium hydroxide if less than 04 M but 01 M or more. Contact with extremely high concentrations of sodium hydroxide can result in severe burns to the eyes skin digestive system or lungs which can lead to permanent damage or death. Mucous membranes in the nose throat lungs and bronchial system may be damaged.
Even tiny doses can cause significant harm. Burns the skin and damages the eyes. The respiratory tract is irritated.
Causes severe burns of eyes skin and mucous membranes. USCG 1999 Reactivity Profile. SODIUM HYDROXIDE SOLUTION refers to an aqueous solution of sodium hydroxide.
Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide. Sodium hydroxide can cause blindness if it gets into your eyes and burns if splashed on your skin. Any spills on your skin and eyes should be flushed with water for 30 minutes.
Large spills and exposure to eyes will also require medical attention after flushing with water. Be sure to read the Medical Safety Data Sheet that comes with your lye or request one from your supplier. Do not under any.
The reaction from mixing of sodium hydroxide and hydrogen peroxide H 2 O 2 yields sodium peroxidea strong oxidizer. This reaction can be violently exothermic and produce a heated mist capable of causing severe burns. Sodium Hydroxide should be considered incompatible with other substances and materials until proven otherwise.
Sodium hydroxide lye and caustic soda all refer to the same compound NaOH which is comprised of 3 single atoms. Sodium oxygen and hydrogen. NaOH is often used to teach about pH in high schools and it can neutralize HCl.
Using some simple supplies you can make homemade NaOH for use in the lab or just to impress your colleagues and students. Although NaOH can be made with or. Sodium hydroxide is soluble in water ethanol and methanol.
This alkali is deliquescent and readily absorbs moisture and carbon dioxide in air. Lye is mixed with water to create a lye solution. Lye solution when mixed with fats and oils will cause a chemical reaction called saponification fancy for soap.
The result of saponification is beautiful handmade soap. In Food Prep. Sodium forms flammable hydrogen and caustic sodium hydroxide on contact with water.
Ingestion and contact with moisture on skin eyes or mucous membranes can cause severe burns. 102 103 Sodium spontaneously explodes in the presence of water due to the formation of hydrogen highly explosive and sodium hydroxide which dissolves in the water liberating more surface. PURUMSODIUM HYDROXIDE EP PELLETSCAUSTIC SODA FLKCAUSTIC SODA MICROPEARLCAUSTIC SODA PEARL OGCAUSTIC SODA MICROPEARL SLY REACH registration number 01-2119457892-27 CAS number 1310-73-2 EU index number 011-002-00-6 EC number 215-185-5 12.
Relevant identified uses of the substance or mixture and uses advised against Identified uses Chemical Chemical Intermediate. Section 15 - Regulatory Information US Federal Regulations TSCA Section 8b. CAS 1310-73-2 is listed on the TSCA inventory.
Answer 1 of 14. If you were foolish enough to put solid NaOH into commercial hydrochloric acid and stir the heat generated would very likely splash a lot of corrosive material around. If you were to mix a dilute aqueous solution of NaOH with a dilute aqueous solution of HCl you would get a di.
Sodium Hydroxide 10 wv LD50 dermal rabbit 14835 mgkg ATE US dermal 14835 mgkg body weight Water 7732 -18 -5 LD50 oral rat 90000 mgkg ATE US oral 90000 mgkg body weight Skin corrosionirritation. Causes severe skin burns and eye damage. 14 Serious eye damageirritation.
Causes serious eye damage. LIQUID CAUSTIC SODA Sodium Hydroxide Diaphragm and Membrane Grades CAS Number. 1310 -73 -2 Synonyms.
Sodium hydroxide caustic soda caustic lye Chemical Formula. Caustic soda solutions are colorless and strongly alkaline. They are not combustible and do not support combustion.
Product Overview Caustic soda is an essential ingredient in many. Sodium hydroxide MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Soda caustic CAS.
40 gmol Chemical Formula. 106462 View Pricing Availability. Download Product Safety Card.
Sodium hypochlorite is used as a bleaching and disinfecting agent and is commonly found in household bleach. The objective is to review critically the epidemiology mechanisms of toxicity clinical features diagnosis and management of hypochlorite poisoning. PubMed was searched from January 1950 to June 2018 using the terms Hypochlorite Sodium.
LYE CAN CAUSE SEVERE CHEMICAL BURNS AND EVEN DEATH IF NOT HANDLED PROPERLY. Lye Uses and Benefits of Sodium Hydroxide. Having become an incredibly versatile and useful ingredient in everything from food preparation to drain cleaning its no wonder that the world produced 60 million metric tons of sodium hydroxide in 2004.
A brief overview of the many uses include. Food It can be. Symptoms from getting sodium hydroxide on the skin or in the eyes include.
Vision loss Home Care. Seek immediate medical help. DO NOT make a person throw up unless told to do so by Poison Control or a health care professional.
If the chemical was swallowed immediately give the person water or milk unless instructed otherwise by a health care provider. Addition of chlorine to cold dilute solution of sodium hydroxide. The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals.
Merck and Co Inc 1996 p. Hazardous Substances Data Bank HSDB Soln of sodium hypochlorite containing 045-050 g of the salt in 100 ml. Prepd by diluting with distilled water a soln of sodium.