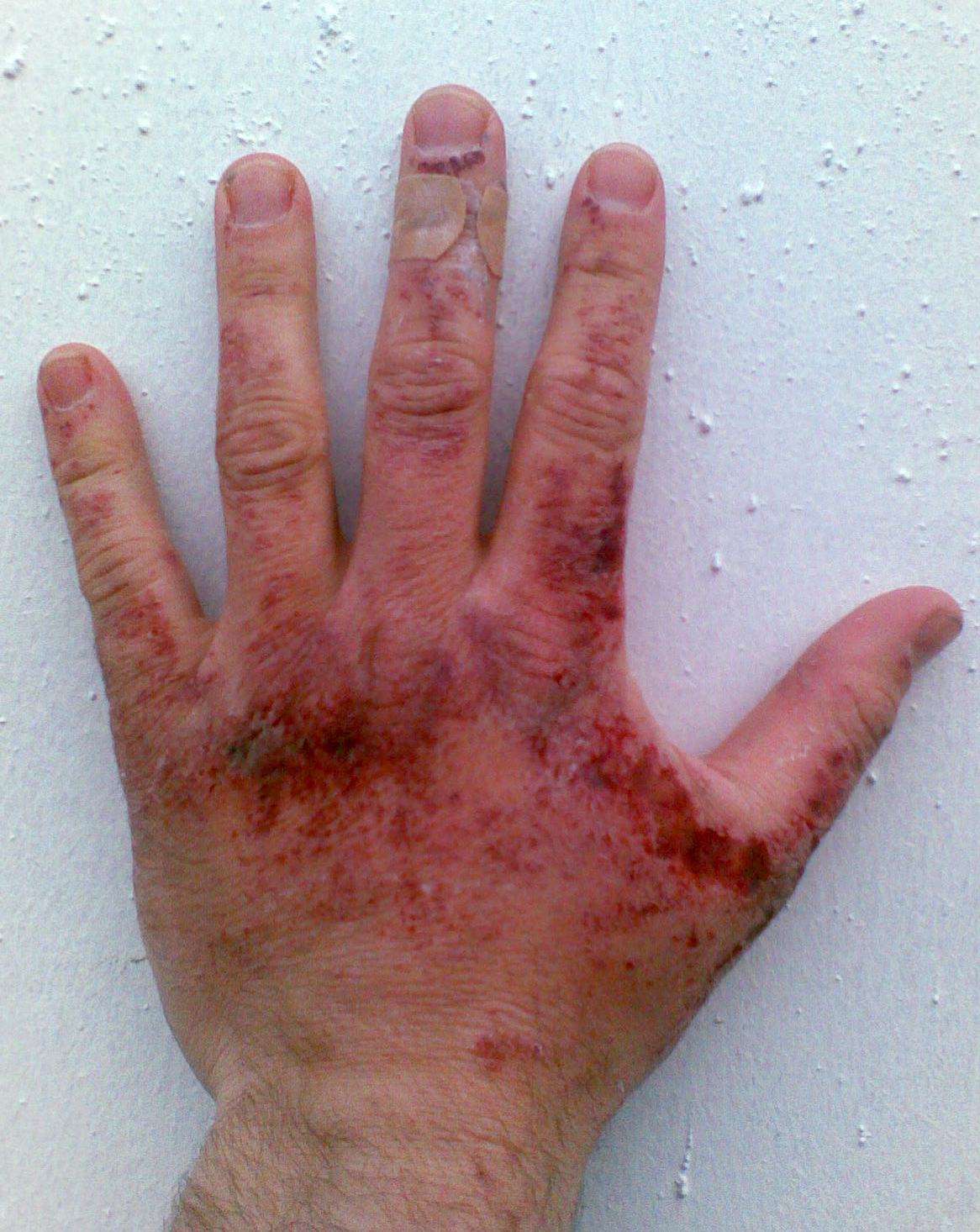
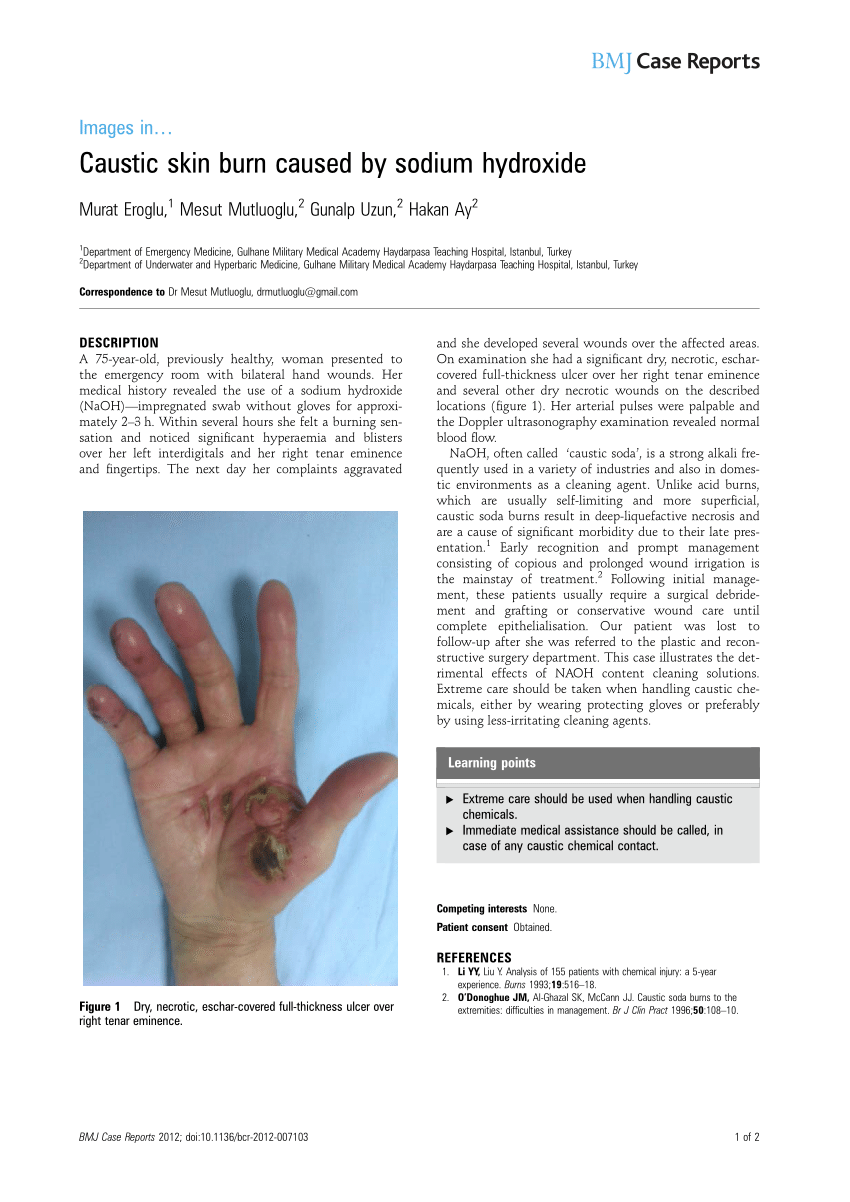
SODIUM HYDROXIDE Caustic Soda is a strong base. Sodium Hydroxide is a HIGHLY CORROSIVE CHEMICAL and contact can severely irritate and burn the skin and eyes with possible eye damage.
Proper NaOH storage guidelines and maintaining workerenvironment safety is always recommended given the chemicals burn hazard.
Sodium hydroxide burn. Sodium hydroxide also known as lye and caustic soda is an. Proper NaOH storage guidelines and maintaining workerenvironment safety is always recommended given the chemicals burn hazard. Sodium hydroxide is often stored in bottles for small-scale laboratory use within intermediate bulk containers medium volume containers for cargo handling and transport or within large stationary.
Sodium hydroxide NaOH is a an odorless white crystalline solid that absorbs moisture from the air. Sodium hydroxide is very corrosive. It can cause irritation to the eyes skin and mucous membrane.
Eye and skin burns. And temporary loss of hair. Workers may be harmed from exposure to sodium hydroxide.
The level of harm depends upon the dose duration and work being done. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents.
Worldwide production in 1998 was around 45 million tonnes. Sodium hydroxide is also the most common base used in chemical laboratories. Sodium hydroxide solvation is highly exothermic can cause splashing to burn.
When handling sodium hydroxide for use especially bulk volumes it should be stored carefully given the chemicals burn hazard. Sodium hydroxide can be stored in bottles for small-scale laboratory use. For use in cargo handling and transport it should be stored in intermediate bulk containers medium volume.
Properties of Sodium Hydroxide NaOH. Sodium Hydroxide is a white translucent crystalline solid. It is commonly referred to as caustic soda due to its corrosive action on many substances it decomposes proteins at room temperatures and may cause chemical burn to human bodies.
SODIUM HYDROXIDE SOLUTION refers to an aqueous solution of sodium hydroxide. Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide phosphorus pentaoxide and with organic and inorganic acid chlorides. Sodium hydroxide lye and caustic soda all refer to the same compound NaOH which is comprised of 3 single atoms.
Sodium oxygen and hydrogen. NaOH is often used to teach about pH in high schools and it can neutralize HCl. Using some simple supplies you can make homemade NaOH for use in the lab or just to impress your colleagues and students.
SODIUM HYDROXIDE Caustic Soda is a strong base. Reacts rapidly and exothermically with acids both organic and inorganic. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas.
Catalyzes the polymerization of acetaldehyde and other polymerizable compounds. These reactions can occur violently for example. Not combustibleUse extinguishing agent suitable for surrounding fire.
Specific Hazards Arising from the Chemical. Contact with water causes violent frothing and spatteringReacts with metals to produce highly flammable hydrogen gas. Sodium hydroxide is soluble in water ethanol and methanol.
This alkali is deliquescent and readily absorbs moisture and carbon dioxide in air. Lye is mixed with water to create a lye solution. Lye solution when mixed with fats and oils will cause a chemical reaction called saponification fancy for soap.
The result of saponification is beautiful handmade soap. In Food Prep. Sodium Hydroxide can affect you when inhaled and by passing through the skin.
Sodium Hydroxide is a HIGHLY CORROSIVE CHEMICAL and contact can severely irritate and burn the skin and eyes with possible eye damage. Contact can irritate the mouth nose and throat. Inhaling Sodium Hydroxide can irritate the lungs.
Sulfuric acid hydrochloric acid sodium hydroxide lime silver nitrate and greater than 5 hydrogen peroxide soloutions. A chemical burn occurs when living tissue is exposed to a corrosive substance such as a strong acid base or oxidizer or a cytotoxic agent such as mustard gas lewisite or arsine. Chemical burns follow standard burn classification and may cause.
Answer 1 of 14. There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances. Great care is needed.
Caustic soda is one of the common names for sodium hydroxide NaOH. It is also known as lye although lye may refer to either potassium hydroxide or sodium hydroxide. Pure caustic soda is sold for making candles or soap.
Impure caustic soda is found in drain cleaner. Because lye is used to make illegal drugs its harder to buy large quantities than in the past. However small containers are.
Answer 1 of 3. Acetil salicylic acid is an organic acid having one carboxilic group which confers the acidity to the acetil salicylic acid and then we can write it as C8H7COOH then a neutralization reaction of the acid with soda NaOH will be C8H7COOH NaOH C8H7COONa H2O or C9H8O4 Na. With caution the treatment using vinegar or weak acid will neutralize the sodium hydroxide and vaporize the chlorine that came as residue from hypochlorite.
T-shirt and cotton sheets that rip easily show the effect of using bleach when washing the clothes. Hot water can increase the effectiveness of bleach since there is the increase of reactivity of those molecules. Symptoms from getting sodium hydroxide on the skin or in the eyes include.
Vision loss Home Care. Seek immediate medical help. DO NOT make a person throw up unless told to do so by Poison Control or a health care professional.
If the chemical was swallowed immediately give the person water or milk unless instructed otherwise by a health care provider. Sodium hydroxide is also used in biodiesel manufacture and as a key component in products that remove blockages from drains. Baking soda actually contains sodium its in the name and its chemical name is sodium bicarbonate where Im sure youve come across it in baking or cooking where it undergoes thermal decomposition at above 70C to release carbon dioxide - which then makes your.
One of the elements that makes the sun burn so brightly is hydrogen. While hydrogen burns really easily on Earth the burning hydrogen on the Sun and other stars is the result of nuclear reactions that rely on istotopes of hydrogen. Rocket Fuel Hydrogen is awesome as a rocket fuel because of all of the energy released.
The hydrogen is compressed into a liquid formH 2 and stored in tanks. A colourless solution is formed consisting of strongly alkalic sodium hydroxide caustic soda and hydrogen gas. This is an exothermic reaction.
Sodium metal is heated and may ignite and burn with a characteristic orange flame. Hydrogen gas released during the burning process reacts strongly with oxygen in the air. A number of sodium compounds do not react as strongly with water but are.
Sodium hydroxide 1310 -73 2 92 Section 3. Hazards Identification Potential Acute Health Effects. Very hazardous in case of skin contact irritant of eye contact irritant of ingestion.
Hazardous in case of skin contact corrosive of eye contact corrosive. Slightly hazardous in case of inhalation lung sensitizer. Liquid or spray mist may.
Metallic sodium reacts with ordinary air to form a thin sodium hydroxide film NaOH. It further absorbs carbon dioxide from the air to form sodium bicarbonate NaHCO 3. In a dry air atmosphere sodium burns by giving away a dense white caustic smoke.
Oxidation of sodium in dry air will result into sodium monoxide Na 2 O. Under two different conditions reaction of sodium with. F Sodium Hypochlorite can irritate and burn the eyes with possible eye damage.
F Inhaling Sodium Hypochlorite can irritate the nose and throat. F Inhaling Sodium Hypochlorite can irritate the lungs causing coughing andor shortness of breath. Higher exposures may cause a build-up of fluid in the lungs pulmonary edema a medical emergency with severe shortness of breath.
F Exposure to Sodium. Sodium methylate is a white amorphous powder. It reacts with water to form sodium hydroxide a corrosive material and methyl alcohol a flammable liquidThe heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts.
It is used to process edible fats and oils and to make other chemicals. Sodium peroxideis spontaneously flammable with aniline benzene organic matter such as paper and wood and diethyl ether. Mixtures with charcoal glycerine certain oils and phosphorus burn.
When a mixture of sodium peroxide and calcium carbide powdered is exposed to damp air spontaneous combustion occurs.