Odium-22 is a radioactive isotope of sodium undergoing positron emission to 22Ne with a half-life of 2605 years. Energy of first ionisation.

While the sampling procedure is easily automated AAS it is inefficient both time and sample volume for routine multielement analysis.
Sodium concentration blood atomic emission. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. It is a soft silvery-white highly reactive metalSodium is an alkali metal being in group 1 of the periodic table. Its only stable isotope is 23 Na.
The free metal does not occur. Any extra sodium may contribute to high blood pressure. Sodium is important for many different functions of the human body.
For example it helps cells to transmit nerve signals and regulate water levels in tissues and blood. Sodium is the sixth most common element on Earth and makes up 26 of the Earths crust. The most common compound is sodium chloride.
During treatment of hyponatremia serum sodium should not be elevated by more than 10 to 12 mEqL in 24 hours or 18 mEqL in 48 hours. In the case of severe hyponatremia where severe neurologic symptoms are present a faster infusion rate to correct serum sodium concentration may be needed. Patients rapidly treated or with serum sodium.
Na in human blood plasma to 24 Na. By measuring the concentration of this isotope the neutron radiation dosage to the victim can be computed. 22 Na is a positron-emitting isotope with a remarkably long half-life.
It is used to create test-objects and point-sources for positron emission tomography. Nuclide Z N Isotopic mass Half-life Decay mode Daughter isotope Spin and. Sodium is a chemical element with atomic number.
By measuring the concentration of this isotope the neutron radiation dosage to the victim can be computed. Sodium-22 is composed of 11 protons 11 neutrons and 11 electrons. Odium-22 is a radioactive isotope of sodium undergoing positron emission to 22Ne with a half-life of 2605 years.
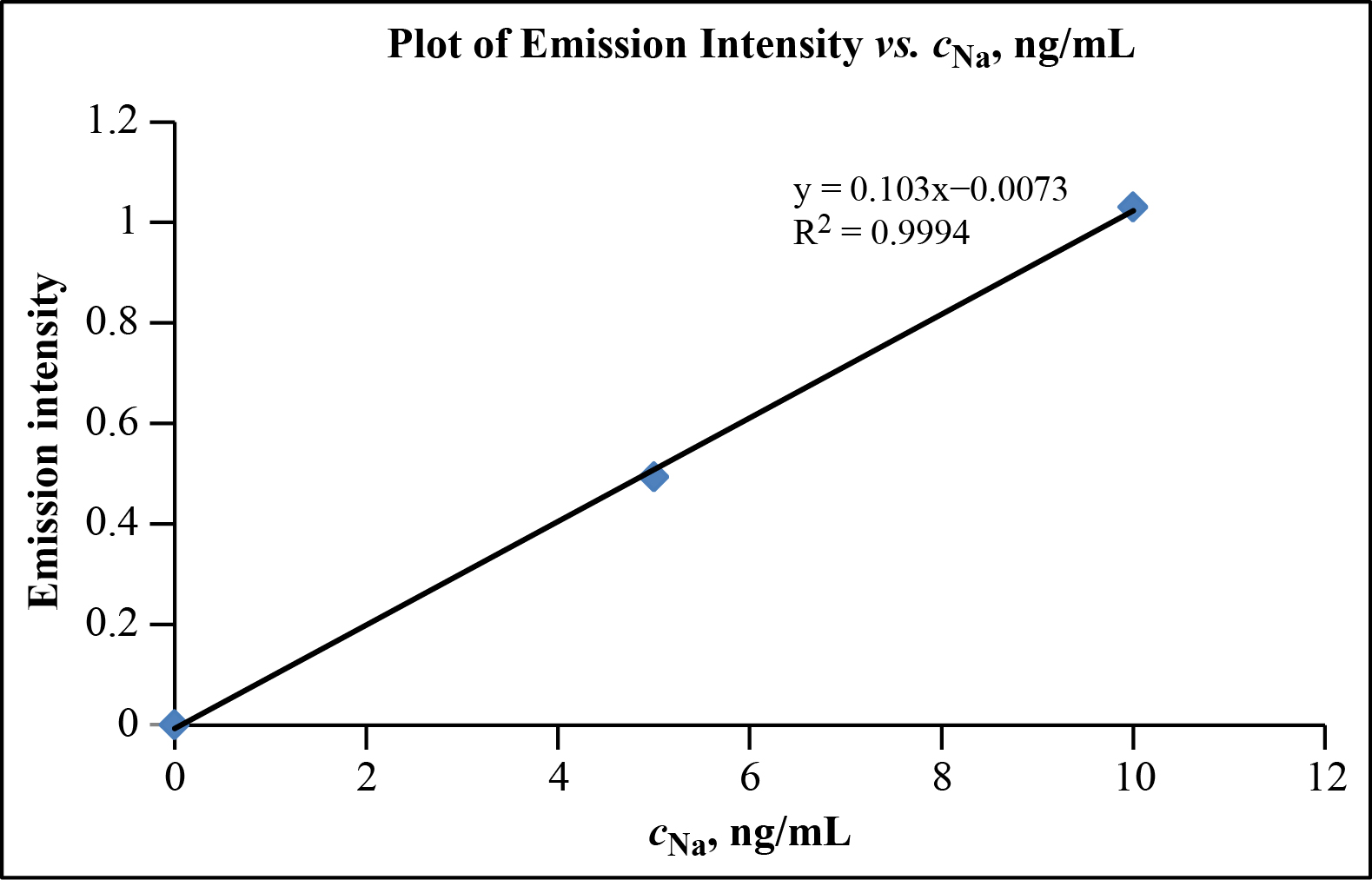
It is also commonly used as a positron source in. Inductively coupled argon plasma atomic emission spectroscopy. For sodium this method has an estimated detection limit of 10 ngmL sample.
The precisionRSD and the recovery are not determined. The working range of this method is 0005 to 20 mgcu m for each element in a 500 liter air sample. Sodium bicarbonate is used for the treatment of metabolic acidosis which may occur in severe renal disease uncontrolled diabetes circulatory insufficiency due to shock or severe dehydration extracorporeal circulation of blood cardiac arrest and severe primary lactic acidosis.
Also is indicated in severe diarrhea which is often accompanied by a significant loss of bicarbonate. COOKBOOKDOC 91096 141 PM Safety Information The Analytical Methods section describes methodologies using a wide variety of potentially hazardous chemicals acids bases organic. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms while atomic absorption spectroscopy measures the light absorbed by atomic absorption.
This light is typically in the visible or ultraviolet region of the electromagnetic spectrum. The percentage is then compared to a calibration curve to determine the amount of material in the sample. Both atomic absorption and emission have been used with the former most widely applied to the analysis of minor soil components.
Detection limits can often be very similar to the concentration found in extraction solutions of natural soils. While the sampling procedure is easily automated AAS it is inefficient both time and sample volume for routine multielement analysis. Flame photometry is one of the branches of atomic absorption spectroscopy.
It is also known as flame emission spectroscopy. Currently it has become a necessary tool in the field of analytical chemistry. Flame photometer can be used to determine the concentration of certain metal ions like sodium potassium lithium calcium and cesium etc.
In flame photometer spectra the metal ions. The classical value of a normal anion gap is considered 124 mEqL when sodium was determined by flame photometry based on the principle of flame atomic emission spectrometry and chloride by a colorimetric assay mercuric-nitrate-thiocyanate colorimetric assay. Since the 1980s ion-selective electrodes for specific ionic species were used for the measurement of serum electrolytes.
However atomic absorption spectroscopy as a modern technique for chemical analysis dates from 1955 when the Lancashire-born scientist Alan Walsh published his significant paper on the potential for AAS in Melbourne Australia. Walshs breakthrough came with the realisation that he needed to be measuring absorption of light rather than emission. Hydrochloric acid HCl was known to the alchemists.
The gaseous element itself was first produced in 1774 by Carl Wilhelm Scheele at Uppsala Sweden by heating hydrochloric acid with the mineral pyrolusite which is naturally occuring manganese dioxide MnO 2A dense greenish-yellow gas was evolved which he recorded as having a choking smell and which dissolved in water to give an acid. AUS-e-TUTE is a science education website providing notes quizzes tests exams games drills worksheets and syllabus study guides for high school science students and teachers. As iodine has a high atomic number 53 compared to most tissues in the body the administration of iodinated material produces image contrast due to differential photoelectric absorption.
Iodine has a particular advantage as a contrast agent because the k-shell binding energy k-edge is 332 keV similar to the average energy of x-rays used in diagnostic radiography 1. When the incident x. Analysis of Vanadium Nickel Sodium and Iron in Fuel Oils using Flame Atomic Absorption Spectrophotometry Elemental analysis of fuel oil is an important step in quantifying its quality.
While ICP-OES and ICP-MS instrumentation may receive more attention when it comes to metals analyses FAAS is a viable option particularly in the petroleum industry. Analysis of Vanadium Nickel Sodium and Iron in Fuel Oils using Flame Atomic Absorption Spectrophotometry Elemental analysis of fuel oil is an important step in quantifying its quality. While ICP-OES and ICP-MS instrumentation may receive more attention when it comes to metals analyses FAAS is a viable option particularly in the petroleum industry.
Electronegativity according to Pauling. 87 gcm-3 at 20C. 0097 nm 2 Isotopes.
Electronic shell Kr 4d 10 5s 2. Energy of first ionisation. Energy of second ionisation.
Vinpocetine is a man-made chemical. Its structure is similar to a substance found in the periwinkle plant. People use vinpocetine as medicine.
We would like to show you a description here but the site wont allow us. Lithium is an element of the alkali-metal group with atomic number 3 atomic weight 694 and an emission line at 671 nm on the flame photometer. Each peach-colored film-coated extended-release tablet contains 300 mg of lithium carbonate.
This slowly dissolving film-coated tablet is designed to give lower serum lithium peak concentrations than obtained with conventional oral lithium dosage. This website uses cookies to help provide you with the best possible online experience. Please read our Terms Conditions and Privacy Policy for information about.
0947 parts per billion ppb whole blood Range. 007 to 23 ppb. Polyaromatic hydrocarbons PAHs - tested for 18 found 9 Pollutants from burning gasoline and garbage.
Accumulates in food chain. 5 of 5 newborns tested Average concentration. 285 parts per trillion ppt blood lipids.
Disposal of Radioactive waste in public domain is undertaken in accordance with the Atomic Energy Safe disposal of radioactive waste rules of 1987 promulgated by the Indian Central Government Atomic Energy Act 1962. Any prospective plan of a hospital that intends using radioisotopes for diagnostic and therapeutic procedures needs to have sufficient infrastructural and manpower resources to.