Citric acid is a weak acid and only produces about 10 chlorine dioxide. This is quite an important symbol in the list of hazard symbols and meanings because it shows that the substance does not burn itself but produce enough oxygen for other inflammable substances to burn.
A mixture of potassium chlorate and sodium amide explodes Mellor 8258.
Sodium chlorate strong or weak. Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO It may also be viewed as the sodium salt of hypochlorous acidThe anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl 5 H. Students will write the formula and determine if compound is a strong or weak electrolyte.
Sodium at standard temperature and pressure is a soft silvery metal that combines with oxygen in the air and forms grayish white sodium oxide unless immersed in oil or inert gas which are the conditions it is usually stored in. Sodium metal can be easily cut with a knife and is a good conductor of electricity and heat because it has only one electron in its valence shell resulting in weak. Strong bases and weak bases are basic compounds that can release hydroxyl ions OH to an aqueous solution.
Although both of them are bases there are several differences between them. The main difference between a strong base and a weak base is that strong bases can completely dissociate to give all available hydroxyl ions to the system whereas weak bases are partially. A mixture of potassium chlorate and sodium amide explodes Mellor 8258.
If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315. The liquid that became the result of this reaction is known as weak sodium hypochlorite solution.
However this process is not efficient and the alternative production method is searched. One of these methods involves calcium hypochlorite extraction using sodium carbonate in order to produce low levels chlorine. This method is commonly used to produce hypochlorite solution for antiseptic in.
Sodium chlorateiii - NaClO 3. In chlorite ion chlorine atom is in the 5 oxidation state. Hypochlorite Chloratei ion OCl-OCl-ion is an unstable ion it exists only in cold states.
When temperature increases it disproportionate to chloride ion and chlorate ion. Negative charge is on the oxygen atom. As chlorine gas other halogens except fluorine also react.
Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion O 2- which is a very strong base with a high tendency to combine with hydrogen ions. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solutionA concentrated solution of sodium oxide in water will have pH 14.
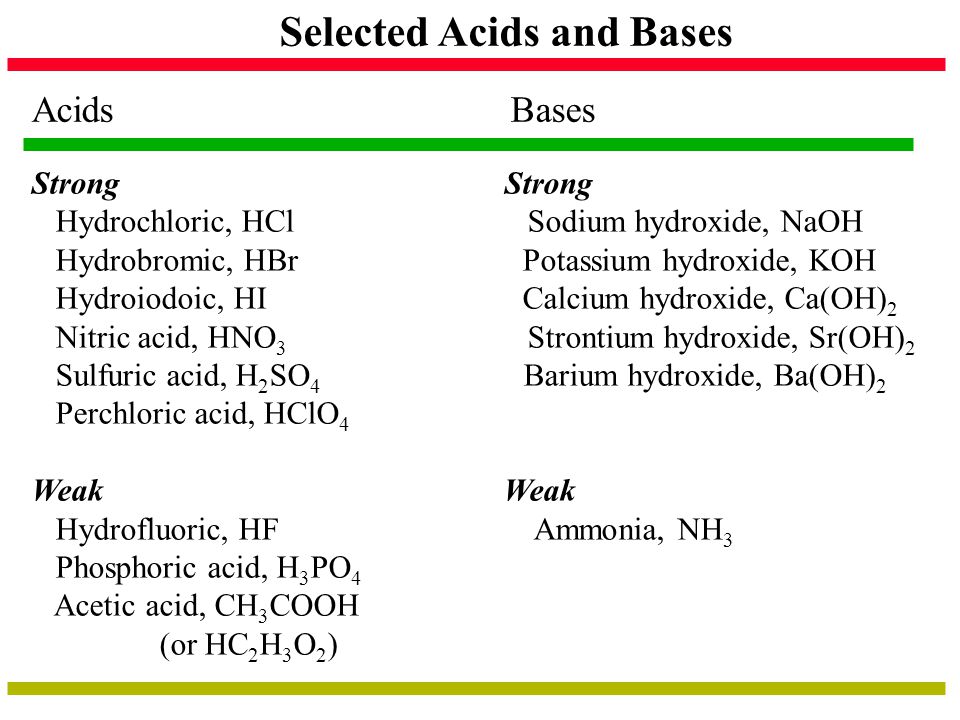
7 strong acids are strong electrolytesHCl HBr HI HNO3 HCIO3 HCIO4 and H2SO4 3 strong bases are strong electrolytesOH KOH and NaOH. The major ionic species present in the solution are sodium and sulfate ions. Sodium sulfate is an ionic compound because it is formed by the complete transfer of electrons from sodium ions to sulfate ions.
When an ionic compound is. Sodium nitrate NaNO3 Potassium sulfate K2SO4 Potassium chloride KCl Potassium chlorate KClO3 Calcium phosphate Ca3PO42 Trisodium orthoborate Na3BO3 NaH2PO2 The H2PO2- ion is derived from H3PO2 acid which is monobasic hence both the H atoms are not replaceable Potassium Perchlorate KClO 4 Acid salts. Acidic salts are a result of the reaction between a strong.
Strong and weak electrolytes Strong Electrolyte. 100 dissociation All water soluble ionic compounds strong acids and strong bases Weak electrolytes Partially ionized in solution Exist mostly as the molecular form in solution Weak acids and weak bases. The impurities are ammonium chloride sodium perchlorate ammonium chlorate and water insolubles.
Menddiratta SK et al. Perchloric Acid and Perchlorates. Kirk-Othmer Encyclopedia of Chemical Technology 1999-2018.
John Wiley Sons Inc. May 13 2005. Hazardous Substances Data Bank HSDB Extremely high purity ammonium perchlorate can be made by the direct.
Specialty polymers Products Specialty polymers. Very strong cost-effective and general purpose chelating agent. The green and strong chelate in our product range.
A safe and readily biodegradable chelating agent that can be used as an alternative for NTAEDTA phosphates and phosphonates especially in cleaning applications. Basic salts are formed by neutralization of a strong base and weak acid. For example the reaction of sodium hydroxide a strong base with acetic acid a weak acid produces water and sodium acetate.
The conjugate acid of the low base makes the salt acidic. What are the properties of an acid. When dissolved in water acids taste acidic conduct electricity and react with metals to create.
Chlorine dioxide is usually produced as a watery solution or gas. It is produced in acidic solutions of sodium chlorite NaClO 2 or sodium chlorate NaClO 3. For large installations sodium chlorite chlorine gas Cl 2 sodium hydrogen chlorite NaHClO 2 and sulphuric or hydrogen acid are used for the production of chlorine dioxide on site.
The emergency oxygen system in a passenger aircraft uses the decomposition of sodium chlorate to produce oxygen. At 760 kPa and 292 K each adult passenger needs about 160 L of oxygen per minute. The equation for the reaction is 2NaClO 3s 2NaCls 3O 2g MNaClO 3 1065 g mol1.
Salts of strong base and weak acid Common Name Soda ash or crystalline sodium are basic in nature with pH value more than 7. Carbonate Molecular Formula Na 2CO 3 10H 2O Common Salt Sodium Chloride NaCl Sodium carbonate can be obtained by heating baking Common salt is formed by the combination of hydrochloric acid and sodium hydroxide solution. Which of the following is a strong acid.
Outcome 1359 DOK 1 A HNO3 B CaSO4 C NH3. Which of the following is a strong acid when mixed in water. Outcome 125 DOK 1 A HClO B HBr C HNO2.
What are the products of the reaction between barium hydroxide and hydrogen chloride. Outcome 12569 DOK 2 A Barium chloride and water B Barium hydroxide and water C Barium. That leaves about 70 leftover sodium chlorite.
Citric acid is a weak acid and only produces about 10 chlorine dioxide. And citric acid can have the taste problem. Luckily the leftover sodium chlorite is also a disinfectant although it is much harsher than chlorine dioxide and not as selective.
With exact concentrations and conditions HCl. Explore the microscopic view of strong and weak acids and bases. Earths atmosphere contains about 20 molecular oxygen O 2 a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world.
The term oxidation was originally used to describe chemical reactions involving O 2. Which of the following compounds is a strong electrolyte. A H2O B CH3OH C CH3CH2OH D HF E NaF.
Which of the following compounds is a weak electrolyte. A HNO3 B NaNO3 C HNO2 D NaNO2 E NaOH. Which of the following compounds is a strong electrolyte.
A H2O D CH3CH2OH ethanol B N2 E KOH C CH3COOH acetic acid E KOH. Which of the following. The most common example is concentrated solution of caustic soda and other strong bases.
This is quite an important symbol in the list of hazard symbols and meanings because it shows that the substance does not burn itself but produce enough oxygen for other inflammable substances to burn. It is therefore important to keep it away from combustible flammable and spontaneously. Some acids and bases are strong wHile others are weak.
Calculate the solubility of potassium cHlorateV if 500 g of the salt is dissolved in 2500cm 3 of water. Solubility Mass of solutesaltsolid x 100 500 x 100 200 g 100g H 2 O Massvolume of watersolvent 2500 c If the solubility of potassium cHlorateV is 5g100g H 2 O at 80 o CHow mucH can dissolve in 5cm 3 of. From an industry perspective there are two main classes of inorganic chemicals.
I alkali chemicals including soda ash which is predominantly sodium carbonate NaCO 3 caustic soda NaOH and liquid chlorine Cl 2 and ii basic inorganic compounds such as aluminum fluoride AlF 3 calcium carbide CaC 2 potassium chlorate KClO 3 and titanium dioxide TiO 2.