
The significant contributions of the editors who have guided preparation of the previous editions is acknowledged. Solubility in water at 25C equals 0559 g 100 mL.
Operating costs for near-complete.
Sodium aluminate solubility in water. Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate is a white crystalline solid having a formula variously given as NaAlO 2 NaAlOH 4 Na 2 OAl 2 O 3 or Na 2 Al 2 O 4Commercial sodium aluminate is available as a solution or a solid. Other related compounds sometimes.
Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
An in vitro oilwater octanolphosphate free aqueous buffer system was used to determine solubilization of aluminum from aluminum borate as hydrophilic or lipophilic complexes and the potential chelation ability of 12 aluminum chelators. Trisodium citrate NN-bis2-hydroxybenzylethylenediamine-NN-diacetic acid cyclohexane-12-diaminotetraacetic acid. The sodium aluminate provides hydroxyl ion OH- needed for improved magnesium reduction without increasing calcium hardness in the treated water.
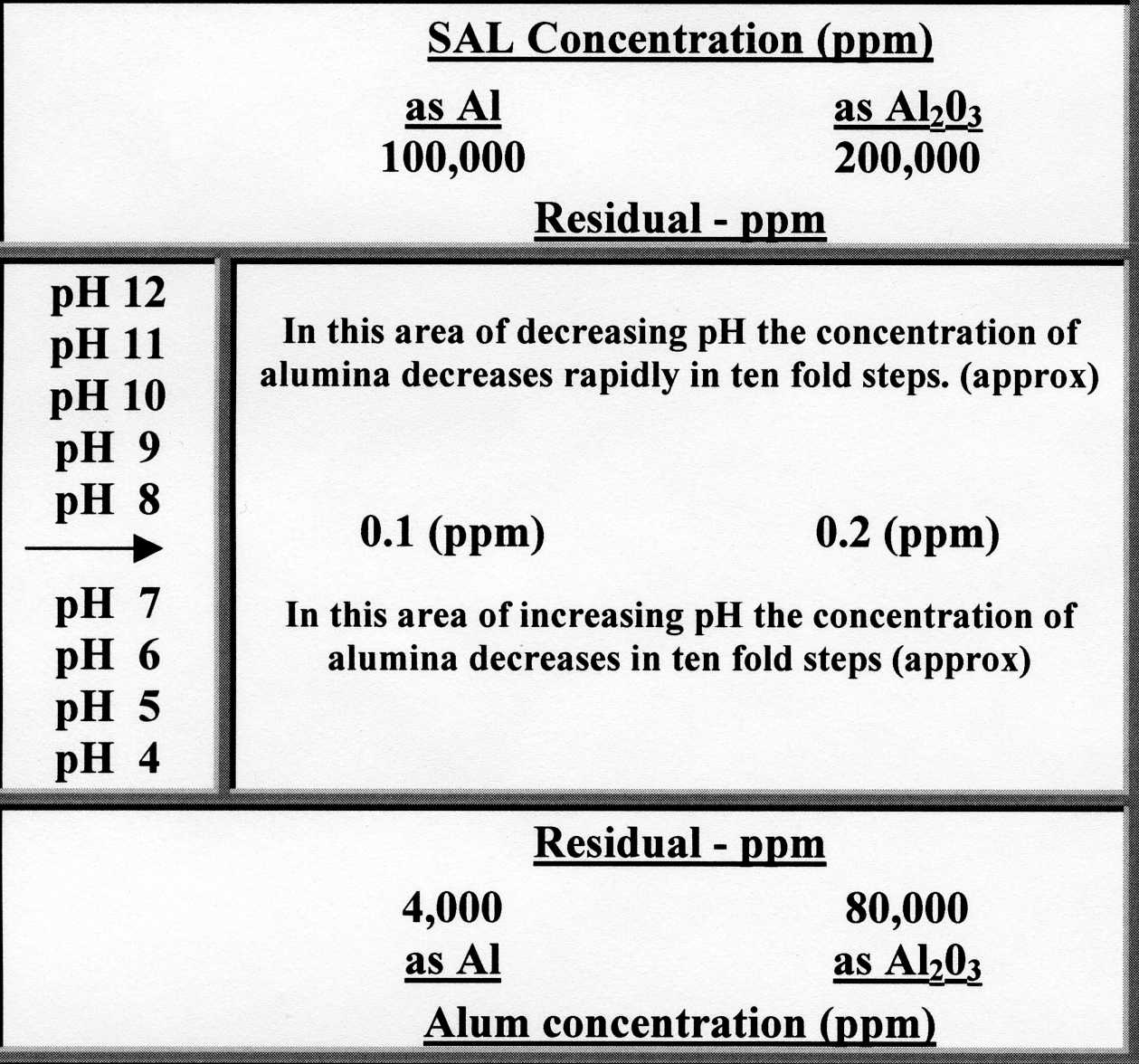
In addition the hydrolysis of sodium aluminate results in the formation of aluminum hydroxide which aids in floc formation sludge blanket conditioning and silica reduction. The reactions are as follows. Na 2 Al 2 O 4.
38-46 Na 2 Al 2 O 4. Variation in pH affects particle surface charge and floc precipitation during coagulation. Iron and aluminum hydroxide flocs are best precipitated at pH levels that minimize the coagulant solubility.
However the best clarification performance may not always. The solubility of sulfate salts running from most to least is. Ammonium magnesium sodium potassium and calcium.
Calcium sulfate found in gypsum-bearing soils attacks only the calcium aluminate phase of the cement matrix to form ettringite calcium sulfoaluminate as follows. Graph 7 Solubility of sodium chloride in aqueous caustic soda solutions 37 Graph 8 Thermal conductivity of aqueous caustic soda solutions 38 Graph 9 Specific conductance of aqueous caustic soda solutions 39 Dilution Calculations 40 Methods of Analysis 43 Determination of the total alkalinity of caustic soda 43 Determination of sodium hydroxide in caustic soda 46 Determination of sodium. The CESR process is simpler and less expensive than other sulfate-removal technologies such as reverse osmosis ion exchange or sodium aluminate addition and is more effective than standard lime precipitation.
This process is a true reduction of total dissolved solids TDS in that all chemicals added for treatment are precipitated during the reactions. Operating costs for near-complete. In water treatment two main types of coagulants are used ie.
Aluminum and iron salts. Type of coagulant formula most common form reaction with water aluminum sulfate Al 2 SO 4 3. 14-18 H 2 O lumps or powder acidic Sodium aluminate NaAlO 2 or Na 2 Al 2 O 4 Powder alkaline Poly-aluminiumchloride Al n OH m Cl 3n-m Solution or powder acidic Ferric sulfate Fe 2 SO 4 39H 2 O Small crystals.
As previously indicated calcium salts appear to have a superior activity compared with most other metal salts but they commonly suffer from a low solubility in water. Calcium formate acts in a manner similar to calcium chloride but high dosages are required and its solubility is considerably less approximately 17 g100 g compared with 75 g100 g at 20C. Ii BeS0 4 and MgS0 4 are readily soluble in water.
Solubility decreases from BeS0 4 to BaS0 4. Due to greater hydration enthalpies of Be 2 ions and Mg 2 ions they overcome the lattice enthalpy factor. Their sulphates are soluble in water.
Carbonates Carbonates of alkaline earth metals are thermally unstable and decompose on heating. The MATERIALS database contains chemical physical visual and analytical information on over 10000 historic and contemporary materials used in the production and conservation of artistic architectural archaeological and anthropological materials. Efflorescence is caused by a number of soluble salts including the sulphate or carbonate compounds of calcium sodium potassium and magnesium.
The salts may originate in the bricks or they may be introduced through the mixing water cement or sand used for the mortar mix or even from the ground on which the bricks were stacked and stored. Solubility in water at 25C equals 0559 g 100 mL. Intestine is the major site of absorption.
The relatively soluble compounds such as sodium fluoride are almost completely absorbed. Fluoride has been detected in all organs and tissues examined. There is no evidence that it is concentrated in any tissues except bone thyroid aorta and perhaps kidney.
High-Aluminum-Affinity Silica Is a Nanoparticle That Seeds Secondary Aluminosilicate Formation. In addn the nanosensors are selective against sodium calcium and magnesium selectivity coeffs. In log10 units of -22 for calcium -20 for sodium and -24 for magnesium three interfering ions found in biol.
The lack of signal overlap between the upconversion nanosensors and GFP a common biol. Fluorescent indicator is demonstrated in confocal microscope images of sensors. In this method the ore is treated with aqueous alkali to form a soluble complex.
For example bauxite an important ore of aluminium is heated with a solution of sodium hydroxide or sodium carbonate in the temperature range 470 520 K at 35 atm to form soluble sodium meta- aluminate leaving behind the impurities iron oxide and titanium oxide. The solubility rules the technique ie when the product of ion concentrations in simple in the solution over the solubility product of the respective solid the precipitation occurs. It is one of the simple methods to purify water.
The chemicals are added to form particles which settle and remove contaminants from water. The treated water is reused whereas the settled portion is. An alkali metal A gives a compound B molecular mass 40 on reacting with water.
The compound B gives a soluble compound C on treatment with aluminium oxide. Identify A B and C and give the reaction involved. A is sodium and B is Sodium Hydroxide.
Because the molecular mass of NaOH is 40. So C is Sodium Aluminate. Al 2 O 3 2NaOH.
This utilizes a scrubber containing high-pH water with calcium magnesium sodium hydroxide and chloride as the scrubbing liquid. CO 2 captured by this water is converted to CaCO 3 and MgCO 3 which are precipitated out of the solution. The precipitates can be filtered washed and dried for reuse as feed material for the kiln to make blended.
GeM is the National Public Procurement Portal. An end-to-end online Marketplace for Central and State Government Ministries Departments Central State Public Sector Undertakings Autonomous institutions and Local bodies for procurement of common use goods services. 120 g of an impure sample of arsenious oxide acting as acidic oxide was dissolved in water containing 75g of sodium bicarbonate and resulting solution was diluted to 250 ml.
25 ml of this. In this process aluminium ore is treated with concentrated sodium hydroxide. Soluble sodium aluminate is formed which is filtered off.
The filtrate on heating with water gives aluminium hydroxide which gives alumina on strong heating. Perrys has been an important source for chemical engineering information since 1934. The significant contributions of the editors who have guided preparation of the previous editions is acknowledged.
Coagulant chemicals that are commonly in use today include iron ferrous or ferric aluminum alum or sodium aluminate and calcium lime. Lime is an attractive chemical for utilization in advanced waste treatment systems and has been selected as the chemical of choice in a number of situations. In some locales lime costs may be lower than either aluminum or iron compounds and lime.