The oxidation of the iron is an exothermic process. Using some simple supplies you can make homemade NaOH for use in the lab or just to impress your colleagues and students.
Silver nitrate sodium chloride — silver chloride and sodium nitrate.
Sodium acetate reaction with water. Sodium Acetate Anhydrous is the anhydrous sodium salt form of acetic acidSodium acetate anhydrous disassociates in water to form sodium ions Na and acetate ions. Sodium is the principal cation of the extracellular fluid and plays a large part in fluid and electrolyte replacement therapies. Sodium acetate anhydrous is used as an electrolyte replenisher in isosmotic solution for parenteral.
Sodium acetate is formed by the reaction of Vinegar 5-8 Acetic acid with sodium carbonate NaHCO 3. In this reaction carbonic acid is formed which is further decomposed by heating produces carbon dioxide and water. CH 3 COOH NaHCO 3 CH 3 COONa H 2 CO 3 H 2 CO 3 CO 2 H 2 O.
Sodium acetate is industrially formed by the reaction of acetic acid with sodium hydroxide in an aqueous. It is concluded that hydrolysis of ethyl acetate reaction in alkaline medium is a shifting order and due to its exothermic nature low reaction temperature promotes high conversion and reaction. The acetate anion is formed from acetic acid and has a chemical formula of CH 3 COO-.
The acetate anion is commonly abbreviated as OAc in formulas. For example sodium acetate is abbreviated NaOAc and acetic acid is HOAc. The acetate ester group connects a functional group to the last oxygen atom of the acetate anion.
Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
The electrolysis of aqueous sodium chloride produces sodium hypochlorite NaOCl a compound of sodium oxygen and chlorine used in large quantities in household chlorine bleach. Sodium hypochlorite is also utilized as an industrial bleach for paper pulp and textiles for chlorination of water and in certain medicinal preparations as an antiseptic and a fungicide. Before the reaction the H atoms and the associated electrons are part of acetic acid molecules.
After the reaction the H protons are part of water molecules while the electrons originally with the H atom are still with the acetate ion. Although not a direct exchange between acetic acid and sodium bicarbonate the hydrogen nucleus has been transferred to a new compound and the electrons. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11.
It is a soft silvery-white highly reactive metalSodium is an alkali metal being in group 1 of the periodic table. Its only stable isotope is 23 Na. The free metal does not occur.
With a Pasteur pipette transferring it to a test tube containing 05 ml of water and 05 ml of ethyl acetate shaking the tube and applying a sample from the top layer to a TLC plate. However we found that applying a spot directly from the reaction mixture is a lot easier and is suitable for the objective. Moreover the extraction process takes some time and dilutes the sample making it more.
Commercial heat packs containing iron and water or supersaturated sodium acetate and cold packs various ammonium salts can be used to show exo- and endothermicity. Heat packs that contain iron and water packets. Exposing the solution to air results in the oxidation of the iron creates rust.
The oxidation of the iron is an exothermic process. Heat packs that contain supersaturated sodium. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions.
The reaction proceeds in two steps. The first reaction is a double. It also illustrates an exothermic reaction and the heat of crystallization.
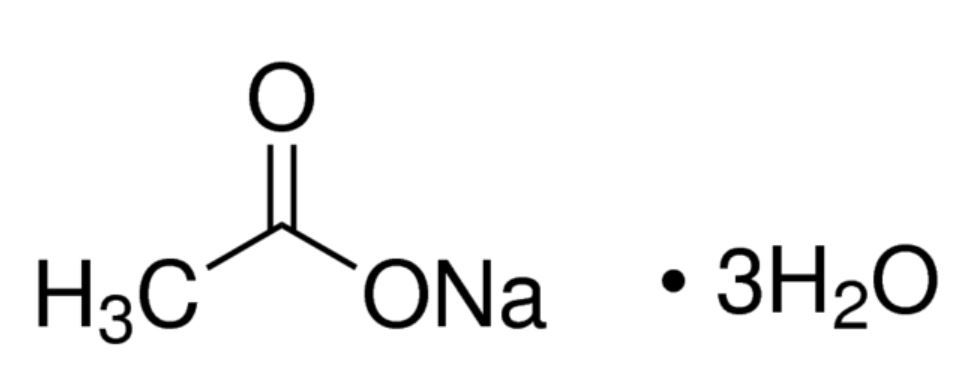
Sodium acetate trihydrate. Hot plate or microwave. Put 160 grams of sodium acetate in a flask and add 30 mL of water.
Put the flask on a hot plate heat it gently and stir until the crystals. Aqueous sulfurous acid H2SO3 and aqueous sodium chloride are formed by the reaction of aqueous sodium sulfite Na2SO3 and aqueous hydrochloric acid HCl. Write a balanced chemical equation for this reaction.
Complete and balance the molecular equation including phases for the reaction of aqueous ammonium bromide NH4Br and aqueous leadII acetate PbC2H3O2. Answer 1 of 14. If you were foolish enough to put solid NaOH into commercial hydrochloric acid and stir the heat generated would very likely splash a lot of corrosive material around.
If you were to mix a dilute aqueous solution of NaOH with a dilute aqueous solution of HCl you would get a di. Sodium bicarbonate baking soda vinegar — carbonic acid and sodium acetate. Sulfuric acid barium hydroxide — barium sulfate and water.
Silver nitrate sodium chloride — silver chloride and sodium nitrate. You can also cause a double replacement chemical reaction when. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
The reaction occurred when a piece of metal salt was dropped in water glass water solution of sodium silicate. C 2 O 4 3 and sodium acetate CH 3 COONa. More accurately these crystals all have water molecules inside them.
Their chemical formulas are CuSO 4 5H 2 O Na 2 S 2 O 3 5H 2 O K 3 FeC 2 O 4 33H 2 O and CH 3 COONa3H 2 O. Dancing Fluorescent Droplets We mixed the. A saturated solution of sodium sulfite in water is mildly basic with an approximate pH value of 9.
Such a solution can undergo crystallization to yield heptahydrate crystals of Na 2 SO 3. In its anhydrous form image provided above sodium sulfite is a white solid. The primary difference between anhydrous Na 2 SO 3 and its heptahydrate is the relative stability of the.
Sodium acetate as shown in the chemical reaction below. NaHCO 3 aq CH 3 COOH aq —- CO 2 g H 2 O l CH 3 COONa aq Stoichiometry can be used to predict the amount of carbon dioxide released in this process. Conservation of mass requires that all atoms that enter a reaction as reactants must exit the reaction in the products.
Consider the example of decomposing water into. Sodium hydroxide lye and caustic soda all refer to the same compound NaOH which is comprised of 3 single atoms. Sodium oxygen and hydrogen.
NaOH is often used to teach about pH in high schools and it can neutralize HCl. Using some simple supplies you can make homemade NaOH for use in the lab or just to impress your colleagues and students. Although NaOH can be made with or.
Besides pure sodium dodecyl sulphate detergent manufacturers usually produce technical grade sodium dodecyl sulphate too a product that consists of approximately 70 sodium dodecyl sulphate and 30 sodium tetradecyl sulphate. This product is generally called sodium lauryl sulphate. Technical grade sodium dodecyl sulphate is used as detergent in dish-washing products main use as additive.
Isopentyl acetate from isopentyl alcohol and acetic acid. Purpose The purpose of this experiment is to synthesize isopentyl acetate 3-methylbutyl acetate via an esterification reaction between acetic acid and isopentyl alcohol 3 -methylbutanol using concentrated sulfuric acid as a catalyst. The product will be washed distilled then.
The result of this initial reaction is two new chemicals. Carbonic acid and sodium acetate. The second reaction is a decomposition reaction.
The carbonic acid formed as a result of the first reaction immediately begins to decompose into water and carbon dioxide gas. Just like carbon dioxide bubbles in a carbonated drink the carbon dioxide that formed as the carbonic acid decomposed. Mixing vinegar and baking soda initiates a chemical reaction that produces carbon dioxide and water.
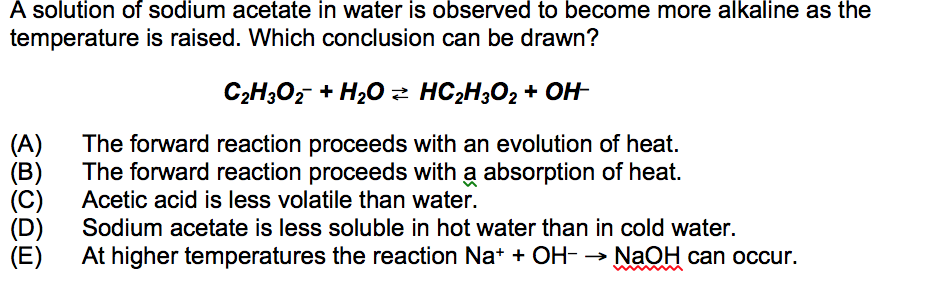
The chemical names of the two ingredients are acetic acid which is vinegar and sodium bicarbonate which is baking soda. In solution the ionic constituents of the salt the acetate ion and the sodium ion separate. Water molecules combine with the acetate ions to form acetic acid and hydroxide ions.
Acetic acid dissociates reversibly into acetate ions and hydrogen ions but only to a very small extent so that the ionic content of the solution is largely sodium and hydroxide ions. Hence the solution exhibits.