
Because sodium is extremely reactive it never occurs in the free state in Earths crust. The reaction proceeds in two steps.
Sodium acetate as shown in the chemical reaction below.
Sodium acetate reaction. For laboratory use sodium acetate is inexpensive and usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid commonly in the 58 solution known as vinegar with sodium carbonate washing soda sodium bicarbonate baking soda or sodium hydroxide lye or caustic soda. Sodium Acetate Anhydrous is the anhydrous sodium salt form of acetic acidSodium acetate anhydrous disassociates in water to form sodium ions Na and acetate ions.
Sodium is the principal cation of the extracellular fluid and plays a large part in fluid and electrolyte replacement therapies. Sodium acetate anhydrous is used as an electrolyte replenisher in isosmotic solution for parenteral. Sodium acetate can be produced from the reaction between acetic acid usually used in the form of vinegar and sodium carbonate usually used in the form of washing soda.
Sodium bicarbonate also known as baking soda or sodium hydroxide also known as caustic soda can be used as an alternative to sodium carbonate in this reaction. Industrially this compound is prepared by reacting acetic. It is concluded that hydrolysis of ethyl acetate reaction in alkaline medium is a shifting order and due to its exothermic nature low reaction temperature promotes high conversion and reaction.
Sodium acetate is prepared by mixing vinegar dilute acetic acid and baking soda sodium bicarbonate and evaporating off the excess water. While acetate salts are typically white soluble powders acetate esters are typically available as lipophilic often volatile liquids. Acetate esters have the general chemical formula CH 3 CO 2 R in which R is an organyl group.
Acetate esters are. Because sodium is extremely reactive it never occurs in the free state in Earths crust. In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form applying electrolysis to fused sodium hydroxide NaOH.
Sodium is an important constituent of a number of silicate materials such as feldspars and micas. In this reaction sodium hydroxide acts as an agent to make the solution alkaline which aluminium can dissolve in. 2 Al 2 NaOH 2 H 2 O 2 NaAlO 2 3H 2.
Sodium aluminate is an inorganic chemical that is used as an effective source of Aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate anhydrous is a white crystalline solid having a formula. Acetate shaking the tube and applying a sample from the top layer to a TLC plate.
However we found that applying a spot directly from the reaction mixture is a lot easier and is suitable for the objective. Moreover the extraction process takes some time and dilutes the sample making it more difficult for the students to perform the TLC in real time. It is very important that students.
Aqueous sulfurous acid H2SO3 and aqueous sodium chloride are formed by the reaction of aqueous sodium sulfite Na2SO3 and aqueous hydrochloric acid HCl. Write a balanced chemical equation for this reaction. Complete and balance the molecular equation including phases for the reaction of aqueous ammonium bromide NH4Br and aqueous leadII acetate PbC2H3O2.
Lacétate de sodium est un composé de formule CH 3 COO-Na. Il est soluble dans leau et constitue une bonne source dions acétate. Sa solution aqueuse est basique en raison de la réaction.
CH 3 COO-Na H 2 O CH 3 COOH HO-Na. Ce composé est utilisé pour faire des expériences simples de solutions aqueuses sursaturées. Lacétate de sodium est.
Sodium acetate and calcium nitrate are examples of suitable salts. These salts are much more soluble in hot water than in cold water. The flexible metal strip is bent back and forth a few times whereupon a white cloud of crystals begins to precipitate.
Within seconds the entire pack is filled up with solid crystalline needles of sodium acetate without any solution left and the temperature. Heat packs that contain supersaturated sodium acetate. These are reusable the packs are boiled to dissolve sodium acetate.
Inside is a metal clicker once the pack cools to room temperature crystallization of sodium acetate an exothermic process is triggered by the clicker which gives the sodium acetate a nucleation point. Cold packs are presumably ammonium salts judging by the stated. A mixture of acetic acid and sodium acetate is acidic because the K a of acetic acid is greater than the K b of its conjugate base acetate.
It is a buffer because it contains both the weak acid and its salt. Hence it acts to keep the hydronium ion concentration and the pH almost constant by the addition of either a small amount of a strong acid or a strong base. If we add a base such as.
Saponification Reaction of Ethyl Acetate and Sodium Hydroxide is an irreversible 2 nd order overall 1 st order with respect to reactants furthermore reaction order decreases and become sequential rather than 2 nd order when equimolecular concentrations of both reactants are used 7-10. Ethyl ethanoate ethyl acetate is an ester. The general method of ester preparation can be summarised as the reversible reaction.
Acid alcohol ester water. Concentrated sulphuric acid is used to catalyse the reaction and to remove water from the right hand side of. The reaction proceeds in two steps.
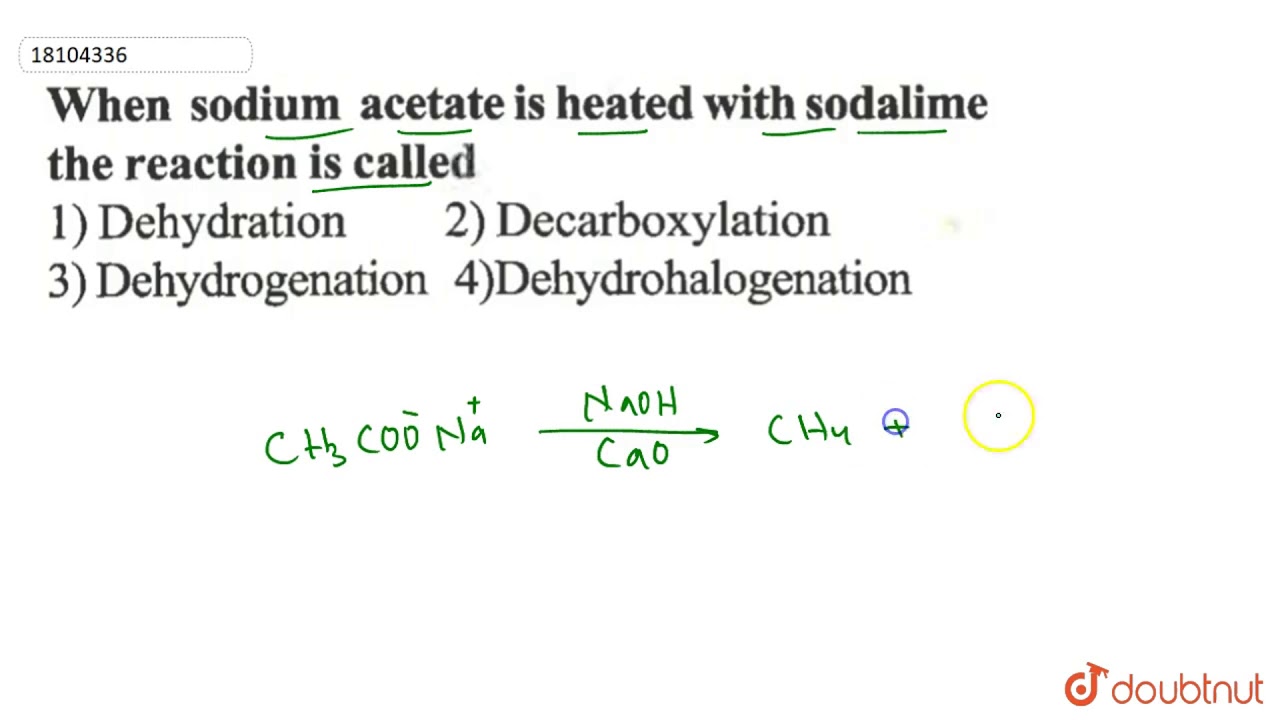
The first reaction is a double displacement reaction while the second reaction is a decomposition reaction. The baking soda and vinegar reaction can be used to produce sodium acetate by boiling off or evaporating all the liquid water. 2021 All rights reserved.
Sodium sulphite Na2SO3 is a salt made from the neutralization reaction between a strong base sodium hydroxide and a weak acid sulphurous acid. Its aqueous solution is therefore distinctly basic in nature with a pH value slightly greater than 7. Is sodium sulfite ionic or covalent.
Sodium sulfite is an ionic compound which crystallizes in a hexagonal crystal lattice in its anhydrous. Sodium bicarbonate baking soda vinegar — carbonic acid and sodium acetate. Sulfuric acid barium hydroxide — barium sulfate and water.
Silver nitrate sodium chloride — silver chloride and sodium nitrate. You can also cause a double replacement chemical reaction when. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
The purpose of this experiment is to synthesize isopentyl acetate 3-methylbutyl acetate via an esterification reaction between acetic acid and isopentyl alcohol 3 -methylbutanol using concentrated sulfuric acid as a catalyst. The product will be washed distilled then characterized using the Thermo Scientific picoSpin 45 NMR spectrometer. Sodium acetate as shown in the chemical reaction below.
NaHCO 3 aq CH 3 COOH aq —- CO 2 g H 2 O l CH 3 COONa aq Stoichiometry can be used to predict the amount of carbon dioxide released in this process. Conservation of mass requires that all atoms that enter a reaction as reactants must exit the reaction in the products. Consider the example of decomposing water into.
In solution the ionic constituents of the salt the acetate ion and the sodium ion separate. Water molecules combine with the acetate ions to form acetic acid and hydroxide ions. Acetic acid dissociates reversibly into acetate ions and hydrogen ions but only to a very small extent so that the ionic content of the solution is largely sodium and hydroxide ions.
Hence the solution exhibits. The first reaction is the acid-base reaction. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda.
The result of this initial reaction is two new chemicals. Carbonic acid and sodium acetate. The second reaction is a decomposition reaction.
A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában.