This is actually how it helps address your stain. Sep 26 2021 Household cleaners can.
Isopropyl ether Di-isopropyl ether is used as a solvent.
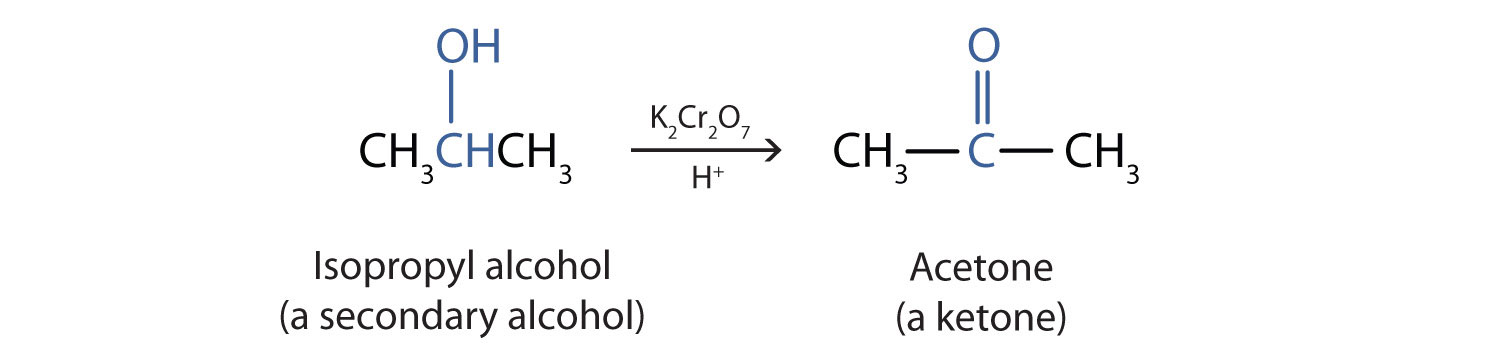
Rubbing alcohol as an oxidizing agent. Isopropyl alcohol has a maximal absorbance at 205 nm in an Ultraviolet-visible spectrum. Isopropyl alcohol can be oxidized to acetone which is the corresponding ketone. This can be achieved using oxidizing agents such as chromic acid or by dehydrogenation of isopropyl alcohol over a.
Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a. Isopropyl alcohol is a secondary alcohol Rubbing alcohol is a solution of 70 isopropyl alcohol and 30 water which is commonly used in sterilizing swabs and disinfectants.
Isopropyl alcohol is a secondary 2º alcohol and is easily oxidized by mild oxidizing agents. 1-Butanol Butyl alcohol 3D. 1-Butanol or butyl alcohol is a four-carbon chain with the OH group on an end.
Vigorous reactions occur with hydrogen palladium nitroform oleum COCl2 aluminum triisopropoxide and oxidizing agents. Reacts explosively with phosgene in the presence of iron salts. Incompatible with acids acid anhydrides halogens and aluminum NTP 1992.
Isopropanol can react with PCl3 forming toxic HCl gas. Logsdon John E Richard A. Loke Isopropyl Alcohol Kirk-Othmer.
The process of alcohol Isopropyl alcohol got the more common name rubbing alcohol in the 1920s because it was used in liniments rubbed into the skin for health and healing. Ethyl alcohol is commonly known as ethanol. For proper disinfecting according to the CDC it must be 60 or more alcohol.
Generally ethanol metabolism occurs at a rate of 10 to 30mgdL per hour. Rubbing alcohol is usually a 70 aqueous solution of isopropyl alcohol. Ethanol is also used in some rubbing alcohol formulations.
When water is removed from an alcohol in a dehydration step the result is either an alkene or an ether depending on the reaction conditions. Primary alcohols are oxidized to aldehydes or carboxylic acids and secondary alcohols are oxidized to ketones. The oxidizing agent can be a metal or another organic molecule.
In the reaction the oxidizing agent is the molecule that is reduced or accepts the electrons. In alcohol oxidation reactions the hydrogen from the alcohol and a hydrogen that is attached to the carbon that has the alcohol attached along with their electrons are removed from the molecule by the oxidizing agent. Removal of the.
We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. This is our newest publication and has been created to support the school technician profession in Scotland. When a tertiary alcohol is exposed to an oxidizing agent no reaction will occur.
Notice that for the primary alcohol that undergoes oxidation that it still retains a hydrogen atom that is attached to the carbonyl carbon in the newly formed aldehyde. This molecule can undergo a secondary oxidation reaction with an oxidizing agent and water to add another oxygen atom and remove the carbonyl. This mechanism is supported by the observation that absolute ethyl alcohol a dehydrating agent.
But it is believed to function similarly to other oxidizing agentsthat is it denatures proteins disrupts the cell wall permeability and oxidizes sulfhydryl and sulfur bonds in proteins enzymes and other metabolites 654. Peracetic acid 026 was. It is generally classified as an oxidizing agent which means it can break down in water and oxygen.
This is actually how it helps address your stain. Additionally it is known to be friendly to all kinds of fabric colored or not. After soaking the sheet in the solution for 24 hours wash it in the machine in a regular washing cycle.
Let the sheet dry naturally. When chemical disinfection of multi-dose vials is desired either isopropyl alcohol 91 or ethyl alcohol 70 is recommended. Many commercially available brands of rubbing alcohol as well as solutions of ethyl alcohol not of USP grade contain denaturants which are injurious to rubber and therefore are not to be used.
It is recommended that chemical disinfection be accomplished by wiping. Yes alcohol is strong enough to kill almost anything including bed bugs and bed bug larvae and eggs. If you use an alcohol dilution of 90 or more you can kill bed bugs on contact.
I like to put alcohol in a spray bottle and spray bed bugs when I spot them. I also spray their hiding places such as nooks and crannies where they lay their eggs. Note however that inhaling.
N-propanol appears as a clear colorless liquid with a sharp musty odor like rubbing alcohol. Vapors are heavier than air and mildly irritate the eyes nose and throat. Density approximately 65 lb gal.
Used in making cosmetics skin and hair preparations pharmaceuticals perfumes lacquer formulations dye solutions antifreezes rubbing. Chlorate Chlorate is a very strong oxidizing agent. Isopropanol isopropanol is the main component in rubbing alcohol.
Isopropyl ether Di-isopropyl ether is used as a solvent. Go To Top J. Jadeite Jade is a popular and valuable gemstone used in China since at least 2950 BC.
Juglone Juglone is used as a natural herbicide. Juncusol Juncusol was isolated from the Juncus. Iodine works by oxidizing cellular components including sulfur-containing amino acids nucleotides and fatty acids and destabilizing the macromolecules that contain these molecules.
It is often used as a topical tincture but it may cause staining or skin irritation. An iodophor is a compound of iodine complexed with an organic molecule thereby increasing iodines stability and in turn. The online variant contains 8.
However since sodium hypochlorite is an oxidizing agent and contains chlorine it can cause irritation of the airway passages as well as burns on the skin. Apr 06 2010 Inhaled substances may cause injury in pulmonary epithelium at various levels of respiratory tract leading from simple symptoms to severe disease. Sep 26 2021 Household cleaners can.
Rubbing alcohol 71 and hydrogen peroxide 3 are commonly used Aug 20 2020 The best natural disinfectants include alcohol hydrogen peroxide vinegar hot water and some essential oils. Disinfectants kill or prevent the growth of bacteria and fungi. Mar 27 2020 Many disinfectants must remain wet on surfaces for an extended time usually 1 to 10 minutes to effectively kill viruses.
Isopropyl alcohol is used in the production of acetone isopropyl halides glycerin and aluminum isopropoxide. Employed widely as an industrial solvent for paints polishes and insecticides. As an antiseptic rubbing alcohol.
And in organic synthesis for introducing the isopropyl or isopropoxy group into the molecule. Being a common laboratory solvent like methanol the exposure risks are. Bleach Alcohol.
Many people see rubbing alcohol and acetone as very benign as cleaning agents. However when these substances touch bleach they create chloroform You know the stuff in movies that kidnappers use to knock people out. 21 According to the CDC chloroform is a probable carcinogen which is the reason it was banned as a drug or for other common uses back in 1976.
Hexadecyltrimethylammonium chloride appears as colorless to pale yellow liquid with an odor of rubbing alcohol. Cetyltrimethylammonium chloride is the organic chloride salt of cetyltrimethylammonium. Polymers Article Polyoligoethylene glycol methacrylate-b- polyvinyl benzyl trimethylammonium chloride Based Multifunctional Hybrid Nanostructures Encapsulating.
Hydrogen Peroxide as Bleaching Agent. H 2 O 2 can also be used as a bleaching agent. Because of its oxidizing properties H 2 O 2 can react directly with double bonds in large organic molecules forming organic peroxides.
H 2 O 2 is used to bleach wood pulp for white paper and the melanin in hair in this way. As a bleaching agent for secondary fibers hydrogen peroxide is appealing. In the usual process a drop of alcohol is added to an unknown stain to dissolve any hemoglobin that may be present followed by rubbing with a swab that has been treated with the Kastle-Meyer reagent.
A drop of hydrogen peroxide is then applied to the swab. If hemoglobin is present the hydrogen peroxide decomposes to yield oxygen that in turn oxidizes the phenolphtalin to. Rubbing alcohol has a shelf life of 2 to 3 years.
- child is always fatigue this will ensure adequate feeding. You may have seen your grandmother dump this stuff on even minor cuts in the past but the truth is that hydrogen peroxide and iodine can cause tissue damage while Mercurochrome has high levels of mercuryall should be avoided. We mix 5 drops of 3.
The MATERIALS database contains chemical physical visual and analytical information on over 10000 historic and contemporary materials used in the production and conservation of artistic architectural archaeological and anthropological materials.