
The corrosion of solid sodium by oxygen also is. Foods such as citrus fruits have acidic properties.
Sodium - sodium - Chemical properties.
Properties of sodium bicarbonate. Decomposition and pH are two common chemical properties of sodium bicarbonate. The concentration of hydrogen ions H in a solution is a chemical property referred to as pH. The pH scale ranges from 0 to 14.
A pH less than 7 indicates an acid a value of 7 is neutral and a value greater than 7 is considered alkaline. A 1 percent molar solution of baking soda in water at room temperature has. Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions.
In patients with renal insufficiency especially those with severe insufficiency such as oliguria or anuria. And in patients receiving corticosteroids or corticotropin since each gram of sodium bicarbonate contains about 12 mEq of sodium. Sodium bicarbonate IUPAC name.
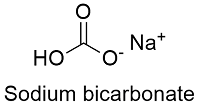
Sodium hydrogencarbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO 3It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3 Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powderIt has a slightly salty alkaline taste resembling that of. Properties of Sodium Bicarbonate. Sodium bicarbonate Chemical formula.
Molecular Weight Molar Mass. A few other important properties of sodium hydrogen carbonate are listed below. NaHCO3 has a white crystalline appearance.
This compound is insoluble in ethanol and slightly. Sodium bicarbonate baking soda is a supplement that provides dietary bicarbonate which can increase serum levels of bicarbonate normally produced by the kidneys and subsequently buffer acid production in the body. The main mechanism of action of sodium bicarbonate is in negating the effects of acidosis.
It provides benefits both in situations of chronic mild acidosis commonly seen in. Sodium bicarbonate also called sodium hydrogen carbonate or bicarbonate of soda NaHCO 3 is a source of carbon dioxide and so is used as an ingredient in baking powders in effervescent salts and beverages and as the main constituent of dry-chemical fire extinguishers. Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis.
It is also employed in. Sodium bicarbonate is a common ingredient in cleaning detergent and degreasing products. In cleaning products sodium bicarbonate can react with vinegar to create a solution that helps unclog drains or remove grime in ovens.
Its slight abrasiveness is extremely efficient for eliminating burnt residues or grease. The chemical properties of sodium bicarbonate can also help enhance the. Sodium bicarbonate is also used as a odor neutralizer cleaning or exfoliating agent and sometimes as a temporary fire extinguisher.
Sodium carbonate is used for body processes or reactions. Sodium bicarbonate is found in our body and is an important element. It helps to regulate and neutralize high acidity levels in the blood.
These are some differences between sodium carbonate and sodium. Interactions do not generally apply to topical use of sodium bicarbonate unless specified. Calcium carbonate-containing antacids should preferably not be taken at the same time as other drugs since they might impair absorption.
Antacids might damage enteric coatings designed to prevent dissolution in the stomach. The bicarbonate ion hydrogencarbonate ion is an anion with the empirical formula HCO 3 and a molecular mass of 6101 daltons. It consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement with a hydrogen atom attached to one of the oxygens.
It is isoelectronic with nitric acid HNO 3The bicarbonate ion carries a negative. SODIUM ALGINATE WITH POTASSIUM BICARBONATE. Show all parts of this monograph.
Prescribing and dispensing information. Management of mild symptoms of dyspepsia and gastro-oesophageal reflux disease. By mouth using chewable tablets.
For Child 611 years under medical advice only 1 tablet to be chewed after meals and at. Sodium bicarbonate reduces the absorption of a number of other drugs taken concomitantly. These include ACE inhibitors captopril enalapril and fosinapril antibacterials and antifungals azithromycin cefaclor cefpodoxime isoniazid itraconazole rifampicin tetracyclines ketoconazole and the quinolone group of antibacterials.
Sodium Bicarbonate 84 wv Solution for Injection contains sodium. This medicinal product contains 2300 mg sodium per ml equivalent to 115 of the WHO recommended maximum daily intake of 2 g sodium for an adult. 45 Interaction with other medicinal products and other forms of interaction.
Caution should be used when administering sodium ions to patients receiving corticosteroids or. Sodium - sodium - Chemical properties. Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOH.
Its chemistry is well explored. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. The corrosion of solid sodium by oxygen also is.
Sodium is a chemical element that has been used by humans since the ancient times. It is the most important metal from a commercial point of view as it is utilized by both organic and inorganic industries. Properties of sodium make it a unique element and here we give you more information about the chemical and physical properties of sodium.
Sodium bicarbonate is a compound used for the symptomatic treatment of heartburn acid indigestion and upset stomach as well as the treatment of metabolic acidosis associated with conditions such as severe renal disease and circulatory insufficiency due to shock. Sodium carbonate was used in soap and also in the process of mummification thanks to its water absorbing and bacteria killing pH control properties. In medieval Europe however sodium carbonate was also used as a cure for headaches and so took the name sodanum from.
Mechanism of Action and Potential Complications of Sodium Bicarbonate Therapy. Bicarbonate is an essential chemical regulating the acid-base balance acting as a buffer Carbon dioxide CO 2 is a major byproduct of energy metabolism in living organisms and a conjugate acidCarbonic anhydrase enzyme facilitates the chemical interaction between CO 2 and water producing carbonic. Sodium bicarbonate NaHCO 3 also called baking soda is a crystalline salt found in a natural mineral form in nahcolite depositsThe science of baking soda has a long and interesting history.
First isolated by Nicolas Leblanc in the 1790s it wasnt until the Solvay process was introduced in the 1860s that industrial-scale production became possible. 1 Today this chemical powerhouse is. Sodium bicarbonate SB administration has been considered an important part of treatment for severe metabolic acidosis in cardiac arrest because based on pathophysiologic considerations normalization of extracellular and intracellular pH was considered a meaningful endpoint of resuscitation.
Correction of metabolic acidosis with SB was recommended by early advanced. Le bicarbonate de sodium Écouter ou carbonate monosodique ou carbonate acide de sodium anciennement bicarbonate de soude a lhydrogénocarbonate de sodium en nomenclature moderne est un compos é inorganique décrit par la formule brute NaHCO 3. Cest un composé ionique blanc de lanion hydrogénocarbonate et du cation sodium qui se présente sous forme de poudres.
La forme ultra. Sodium Chloride is a metal halide composed of sodium and chloride with sodium and chloride replacement capabilities. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function.
Acids and bases have different physical properties 1. One property of acids is a sour taste although laboratory chemicals shouldnt be tasted. Foods such as citrus fruits have acidic properties.
Vinegar is acetic acid and it is also sour. Acids are also corrosive and change litmus paper to red. A property of bases is that the chemicals feel slippery.
Household bleach is a common base. Baking soda is a powdered chemical compound called sodium bicarbonate and vinegar includes acetic acid. These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below.
NaHCO 3 aq CH 3 COOH aq —. The reaction of baking soda sodium bicarbonate and vinegar acetic acid is as follows. Note that all of this occurs in an aqueous ie water-based solution with the water typically provided by the vinegar as it is 5 acetic acid and 95 water.
The sodium bicarbonate dissociates into a sodium cation Na and a bicarbonate anion HCO 3-. The charges arise because single valence.