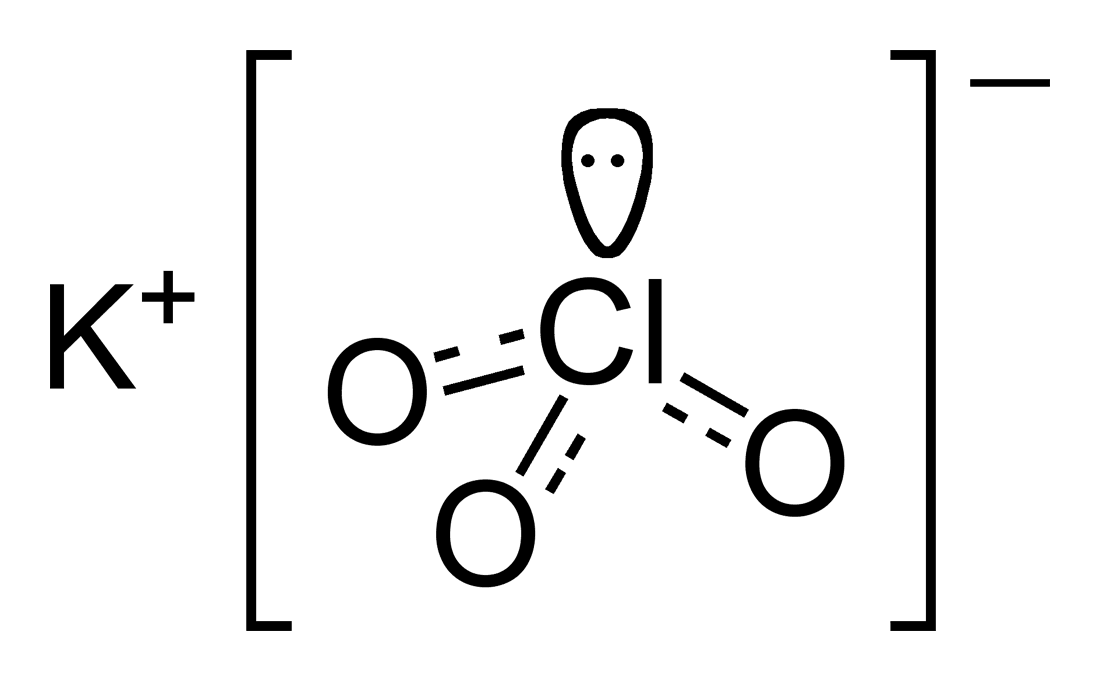
It is a strong oxidizing agent and its most important application is in safety matches. Name the following compounds include Roman Numerals when necessary.
Cation and on the anion.
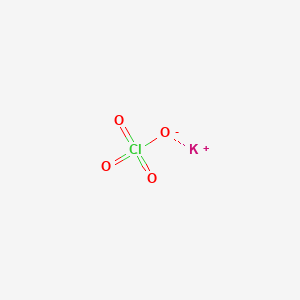
Potassium perchlorate name formula. Perchlorate is the inorganic salt with the chemical formula KClO 4 and is a very common oxidizer in pyrotechnics. Pyrotechnicians use Potassium Perchlorate for making colorful stars and flash powder. Description Additional information Reviews 12 Description.
Composition based on Potassium. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. After sodium chlorate it is the second most common chlorate in industrial use.
It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been. Perchlorate ions are somewhat toxic to the thyroid gland.
Most perchlorates are colorless solids that are soluble in water. Four perchlorates are of primary commercial interest. Ammonium perchlorate NH 4 ClO 4 perchloric acid HClO 4 potassium perchlorate KClO 4 and sodium perchlorate NaClO 4.
Perchlorate is the anion resulting from. 2 Write the name for each of the following compounds. NH4NO2 ammonium nitrite 51.
K2SO3 potassium sulfite 42. MgF2 magnesium fluoride 52. Cu2S copper I sulfide 43.
BaCIO32 barium chlorate 53. MnClO44 manganese IV perchlorate 44. Al2S3 aluminum sulfide 54.
ZnBr2 zinc bromide 45. SnSO42 tin IV sulfate 55. The positive ion cation is written first in the name.
The negative ion anion is written second in the name. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. Parentheses and a subscript are not used unless more than one.
Name or write the formula for the following Type II polyatomic ionic compounds iron III bromate NiMnO33 copper I cyanate CrSO5 plumbous perchlorate SnC2H3O24 mercury I bicarbonate Cu2SO5 antimony III perphosphate MnCO4 arsenic V hypophosphite AuNO3 manganese II carbonate SbPO3 copper I sulfate HgHCO3. Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation.
Sodium perchlorate appears as white crystalline solid. Noncombustible but will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result.
Prolonged exposure to fire or heat may result in an explosion. Used in chemical analysis and in explosives. Potassium Perchlorate KCN Potassium Cyanide KH2PO4 Monopotassium Phosphate KHC8H4O4 Potassium Hydrogen Phthalate KHCO3 Potassium Hydrogencarbonate KIO3 Potassium Iodate KMnO4 Potassium Permanganate KNO3 Potassium Nitrate KOH Potassium Hydroxide KrF2 Krypton Difluoride KSCN Potassium Thiocyanate Li2CO3 Lithium Carbonate Li2O Lithium Oxide Li2SO4 Lithium Sulfate.
Know that it is 1 and perchlorate is an anion so it always has a negative charge. You can use this same method to determine the correct subscript when you are writing a chemical formula based on a name. Write the formula for magnesium bromide.
This is a compound containing magnesium and bromine ions- Mg2 and Br -. Long before chemists knew the formulas for chemical compounds they developed a system of nomenclature that gave each compound a unique name. Today we often use chemical formulas such as NaCl C 12 H 22 O 11 and CoNH 3 6 ClO 4 3 to describe chemical compoundsBut we still need unique names that unambiguously identify each compound.
Sodium chloride and potassium chloride. In the event such products are used goggles should be worn at all times. Notes about the Materials The list of ions was based on a dry lab from JA.
Beran - Laboratory Manual for Principles of General Chemistry. Each card has the name in both the old system and the stock system and the charge of the ion printed on the card. All of.
Write the formula for potassium chloride. The name tells you there are potassium K and chloride Cl. Perchlorate Sr2 strontium SO 3 2 sulfite IO 3 iodate Ba2 barium HSO 3 hydrogen sulfite MnO 4 permanganate Al3 aluminum SO 4 2 sulfate NO 2 nitrite Sn2 tinII HSO 4 hydrogen sulfate NO 3 nitrate Sn4 tinIV S 2O 3 2 thiosulfate OH hydroxide Pb2.
Name _____ Date _____ Period _____ Ionic_Covalent Names. Chapter 9 Honors Chemistry Ionic Covalent Compound Naming Race First identify whether these compounds are ionic or covalent. Then use the correct formula writing rules to write the correct chemical formulas for each compound.
Compound Name Type of Compound. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium. Cation and on the anion.
For example if we were to name calcium nitrate we would start with realizing that the ions involved are Ca2 and NO3- so the formula has to be CaNO32. Note that we do not indicate any charges ononaround the formula. In addition to naming the polyatomic anions from the flashcards you need to know that.
Polyatomic Ionic Compounds 1. Name the following compounds include Roman Numerals when necessary. Na 2SO 4 sodium sulfate AℓPO 4 aluminum phosphate Aℓ C ℓO 4 3 aluminum perchlorate AsPO 3 arsenic III phosphite NiOH 3 nickel III hydroxide AgBrO 3 silver bromate PbIO 3 2 lead II iodate K 3P potassium phosphide HgCN mercury I cyanide MgIO 4.
K2Cr2O7 potassium dichromate 6. KHCO3 potassium bicarbonate 7. Cu2CO3 copper I carbonate 8.
FeNO22 iron II nitrite 9. AgNO3 silver nitrate 10. BaClO42 barium perchlorate 11.
PbCO32 lead II carbonate 12. CdOH2 cadmium hydroxide 13. SnSO4 tin II sulfate 14.
ZnHCO32 zinc bicarbonate 15. Hg2NO22 mercury I nitrite 16. NH42CrO4 ammonium chromate 17.
SnCN2 tin II cyanide. Students enrolled in Dr. Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for the common cations and anions listed below.
The name for an ionic hydrate is derived by adding a term to the name for the anhydrous meaning not hydrated compound that indicates the number of water molecules associated with each formula unit of the compound. The added word begins with a Greek prefix denoting the number of water molecules see Table 5 and ends with hydrate For example the anhydrous compound copperII. Chemical Formula - Indicates the number and type of atoms in the base unit of a compound.
Type of compound Base unit Ionic Formula unit fu Molecular Molecule Valence Electrons - Electrons in the outermost shell of an atom The only e s involved in bonding and chemical reactions. For the S- P-blocks. Valence e Group number Ionic Compounds.
Stearoyl-CoA Desaturase 1 Inhibitor MF-438 CAS 921605-87-0 is a cell-permeable inhibitor of Stearoyl-CoA Desaturase 1 SCD1. IC50 of 23 nM. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO.
Perchlorate HPO 4 2 monohydrogen phosphate MnO 4 permanganate H 2PO 4 dihydrogen phosphate C 2H 3O 2 -acetate OAc HSO 3 hydrogen sulfite bisulfite C 2O 4 2 oxalate There are some regularities in the names of these polyatomic ions. Thio-implies replacing an oxygen with a sulfur. 2SO 4 sulfate 2S 2O 3 thiosulfate OCN cyanate SCN thiocyanate b.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. If you know the correct spelling of the name of the product you wish to review then use the Search feature on the web browser to locate the name in the list. You may also click on the letter from the alphabetical table to go directly to the beginning of that alphabetic section.