
2 NH 4 ions and 1 HSO 4 2-ion. 2 NH 4 ions and 1 HSO 4 2-ion.
Tin IV nitrite SnNO24 16.
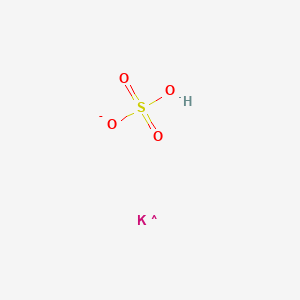
Potassium hydrogen sulfate. Potassium hydrogen sulfate also known as potassium bisulfate KHSO 4 is readily produced by reacting K 2 SO 4 with sulfuric acid. It forms rhombic pyramids which melt at 197 C 387 F. It dissolves in three parts of water at 0 C 32 F.
The solution behaves much as if its two congeners K 2 SO 4 and H 2 SO 4 were present side by side of each other uncombined. An excess of ethanol. Potassium is the major cation positive ion inside animal cells while sodium is the major cation outside animal cells.
The concentration differences of these charged particles causes a difference in electric potential between the inside and outside of cells known as the membrane potential. There are several treatment options for removing hydrogen sulfide and sulfate from household water. The selection of the type and size of a treatment system depends on the concentration of hydrogen sulfide andor sulfate in the water.
Whether you need a point-of-entry whole house treatment or point-ofuse treatment at one faucet supplying water for drinking and cooking system. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force.
Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash the ashes of plants from which its name derives. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes.
We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Suggested Citation4 WaterInstitute of Medicine. Dietary Reference Intakes for Water Potassium Sodium Chloride and SulfateWashington DC.
POTASSIUM PERSULFATE is an oxidizing agent. Noncombustible but accelerates the burning of combustible material. Potassium persulfate plus a little potassium hydroxide and water released sufficient heat and oxygen to ignite a polythene polyethylene liner in a.
The University of Georgia Agricultural and Environmental Services Laboratories offer soil salinity testing to help farmers and the general public diagnose and manage problems associated with soil salinity. By definition a saline soil contains excess soluble salts that reduce the growth of most crops or ornamental plants. This publication discusses soil salinity testing data interpretation.
What is the correct name for the ionic compound Li 3 PO 4. The formula unit for the ionic compound ammonium hydrogen sulfate consists of which of the following. 2 NH 4 ions and 1 SO 4 2-ion.
2 NH 4 ions and 1 HSO 4 2-ion. 1 NH 4-ion and 1 HSO 4 ion. 1 NH 4 ion and 1 HSO 4-ion.
1 NH 4 ion and 1 SO 4. Sulfate Sodium phosphate Sodium nitrate NH 4 Ammonium chloride Ammonium carbonate Ammonium hydroxide Ammonium sulfate Ammonium phosphate Ammonium nitrate KPotassium chloride Potassium carbonate Potassium hydroxide Potassium sulfate Potassium phosphate Potassium nitrate Ca2Calcium chloride Calcium carbonate Calcium hydroxide Calcium sulfate. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
Ionic bonds are atomic bonds created by the attraction of two differently charged ionsThe bond is typically between a metal and a non-metal. The structure of the bond is rigid strong and often crystalline and solid. Ionic bonds also melt at high temperatures.
The chemical reaction in this demonstration is between the hydrogen peroxide and a solution of potassium iodide and dishwashing detergent that captures the gases to make bubbles. 50-100 ml of 30 hydrogen peroxide H 2 O 2 solution Note. This hydrogen peroxide solution is much more concentrated than the kind youd generally purchase at a pharmacy.
LJ liquid junctionValue obtained using a cell which included a liquid junction potential. NHE normal hydrogen electrode SCE saturated calomel electrode See more information about how these numbers were calculated. Reference 7 suggests that the electrolyte filling solution should have sulfate concentrations higher than 001 M and silver ion concentrations between 0001 1 mM.
The composition of the oxidizing agent Oxone is 2KHSO 5. K 2 SO 4. The active component potassium monopersulfate KHSO 5 potassium peroxomonosulfate is a salt from the Caros acid H 2 SO 5.
The use of Oxone has increased rapidly. Reasons for this are the stability the simple handling the non-toxic nature the versatility of the reagent. Potassium salts in the form of saltpetre potassium nitrate KNO 3 alum potassium aluminium sulfate KAlSO 4 2 and potash potassium carbonate K 2 CO 3 have been known for centuriesThey were used in gunpowder dyeing and soap making.
They were scraped from the walls of latrines manufactured from clay and sulfuric acid and collected as wood ash respectively. Sulphur is a multivalent non-metal abundant tasteless and and odorless. In its native form sulphur is a yellow crystalline solid.
In nature it occurs as the pure element or as sulfide and sulfate minerals. Although sulphur is infamous for its smell frequently compare to rotten eggs that odor is actually characteristic of hydrogen sulphide. Consider amount of potassium from all sources when determining dose of drug and do not exceed maximum age-appropriate recommended daily amount of potassium.
In patients with moderate renal impairment eGFR 30 mLmin173 m 2 to 60 mLmin173 m 2 start at low end of dose range and monitor serum potassium phosphorus calcium and magnesium concentrations. Potassium is a shortfall nutrient. Potassium is 1 of the 4 major shortfall nutrients in the American diet according to the 2010 Dietary Guidelines for Americans Advisory Committee Potassium requirements increased substantially in 2004 when the adequate intake was established at 4700 mgd It is difficult for most Americans to achieve these levels of intake.
The first number under the column headed RQ is the reportable quantity in pounds. The number in parentheses is the metric equivalent in kilograms. Potassium carbonate is commercially prepared with the help of a reaction involving carbon dioxide and potassium hydroxide.
In an alternative process in the presence of an organic amine potassium chloride is treated with carbon dioxide to give potassium bicarbonate which is then calcined to provide potassium carbonate. Comment on the solubility of potassium carbonate in water. The effect of acidemia on enhancing cellular K loss is not related to direct potassiumhydrogen H ion exchange but rather via action on transporters which normally regulate skeletal muscle pH.
The decrease in extracellular pH reduces the rate of sodium-H exchange and inhibits sodium Nabicarbonate HCO 3 cotransport. The fall in intracellular Na reduces Na-K. Bubbling SO 2 F 2 gas into a solution of olefin 30 aqueous hydrogen peroxide and 2 M aqueous potassium carbonate in 14-dioxane at room temperature for 1 h provides the corresponding epoxides in good to excellent yields.
This inexpensive mild and highly efficient epoxidizing system is suitable to a variety of olefinic substrates including electron-rich and electron-deficient ones. Ferrous sulfate is an iron supplement used to prevent or treat iron deficiency anemia. Generic Name Ferrous sulfate anhydrous Commonly known or available as Ferrous sulfate DrugBank Accession Number DB13257 Background.
Iron deficiency anemia is a large public health concern worldwide especially in young children infants and women of childbearing age. 4 This type of anemia occurs. Potassium nitrate KNO3 11.
Mercury I cyanide Hg2CN2 12. Iron II acetate FeC2H3O22 13. Aluminum dichromate Al2Cr2O73 14.
Silver sulfate Ag2SO4 15. Tin IV nitrite SnNO24 16. Strontium chlorate SrClO32 17.
Cesium hydrogen sulfite CsHSO3 18. Mercury II phosphite Hg3PO32 19. Iron III oxalate Fe2C2O43 20.