
Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. One is that chloride ammonia and some heavy metals have been known to interfere with PeCOD readings and provide inaccurate results.
If H is the positive ion the compound is an acid as we will see later on page 6 The positive ion cation may be a monatomic metal ion such as.
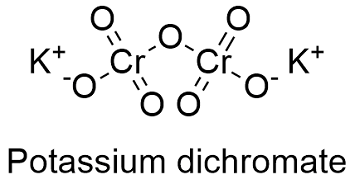
Potassium dichromate chemical formula. Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright red-orange color.
The salt is popular in the laboratory because it is not. Potassium dichromate primarily affects the respiratory tract causing ulcerations shortness of breath bronchitis pneumonia and asthma but can also affect the gastrointestinal tract liver kidneys and immune system. This substance is a known human carcinogen and is associated with an increased risk of developing lung cancer and cancer of the.
The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol. It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three oxygen atoms as well as a bridging oxygen atom. Potassium dichromate or anhydrochromate is prepared by adding to the neutral yellow chromate of potassium in solution a moderate quantity of one of the stronger acids.
Potassium permanganate is commercially prepared by mixing solution of potassium hydroxide and powdered manganese oxide with oxidizing agents like potassium chlorate. Potassium carbonate 2K CO 3 2- K 2 CO 3. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species.
B the chemical formula of the compound appears after the arrow. Sodium sulfate Na 2 SO 4. Potassium carbonate K 2 CO 3 2.
The chemical formula calculator also contains the names of a range of. Most commonly a 025 N solution of potassium dichromate is used for COD determination although for samples with COD below 50 mgL a lower concentration of potassium dichromate is preferred. In the process of oxidizing the organic substances found in the water sample potassium dichromate is reduced since in all redox reactions one reagent is oxidized and the other is reduced forming Cr 3.
Potassium chromate produced the greatest disruption of the skin structure as determined by HE staining followed in order by sodium chromate potassium dichromate and chromium nitrate. This indicates that hexavalent chromium elicited greater toxicity to the skin compared to trivalent chromium. Potassium dichromate Revision Date 19-Jan-2018 Specific Hazards Arising from the Chemical The product causes burns of eyes skin and mucous membranes.
Contact with combustibleorganic material may cause fire. May ignite combustibles wood paper oil clothing etc. Do not allow run-off from fire-fighting to enter drains or water.
List of chemical compounds. Preparation of solutions calculator is a useful tool which allows you to calculate how many solid chemicals or stock solutions you will need to prepare the desired solution. Periodic table of the elements.
Compound name Molecular formula Molar mass gmol Density Range of. 025 N Potassium Dichromate 12259 gm K 2 Cr 2 O 7 dried at 103 0 C 1 Liter DW 01 N Ferrous Ammonium Sulphate 392 gm Fe NH 4 2 SO 4 26H 2 O 20 ml ConcH 2 SO 4 1 Ltr DW Glass beads. Procedure Wash all the apparatus with distilled water Dry the round bottom flask by keeping in oven for 10-15 minute Follow the steps as per protocol mentioned below.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Chemical Formula Nomenclature Practice. Complete these in lab and on your own time for practice.
You should complete this by Sunday. Use the stock form for the transition metals. Give the formula for the following.
Sulfur dioxide SO2_ 2. Sodium thiosulfate ____Na2S2O3_____ 3. Potassium PermanganatePotassium permanganate is an inorganic chemical compound with the formula KMnO4It is a salt consisting of K and MnO4 ions.
Formerly known as permanganate of potash or Condys crystals it is a strong oxidizing agentIt dissolves in water to. A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols numbers and sometimes also other symbols such as parentheses dashes brackets commas and plus and minus signs. In other words a chemical formula represents the ratio of atoms per element that.
It is highly toxic and flammable it is also cancerogenic. It is used mainly in making strong cleaning solutions. It is a good water.
Chemical Formulae and Equation Calculation. Molar volume. Molar volume.
Molar mass. Number of particles. Mole of particles.
Mass of particle in gram molar mass. Avogadro Constant. Volume of Gas.
For Solid liquid or gas. Mass of subtance number of mole molar mass. Molar mass RAMRMMRFM in gram.
For gas only volume of gas. Chemical oxygen demand or COD is a test that measures the amount of organic compounds in water. More specifically the test is a process of decomposing pollutants in water after two hours of boiling the water in a solution of potassium dichromate.
If the COD is high the amount of pollution in the test sample is high. The COD test involves a blank which is a sample made by adding. Im glad you found your way here for chemistry help because youre in the right spot to be enlightened.
By the way if its not actually the holidays and I havent updated this recently sorry about that. Here are the rules for using this free website. I promise that I wont take your.
Chemical or scientific names are used to give an accurate description of a substances composition. Even so you rarely ask someone to pass the sodium chloride at the dinner table. Its important to remember that common names are inaccurate and vary from one place and time to another.
Therefore dont assume that you know the chemical composition of a substance based on its. 3 chromium III dichromate BaC ℓO 2 barium hypochlorite PbC ℓO 2 4 lead IV chlorite PbSO 3 2 lead IV sulfite 2. Write the chemical formula for the following ionic compounds.
Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium. Long before chemists knew the formulas for chemical compounds. Sr 2 strontium.
Some metals form positive ions in more than one oxidation state. One of the earliest methods of distinguishing between these ions used the suffixes -ous and -ic added to the Latin name of the element to represent the lower and higher oxidation states respectively. Fe 2 ferrous.
Fe 3 ferric. Potassium dichromate K2Cr2O7 is an oxidising agent that causes alcohols to oxidise. The exact reaction however depends on the type of alcohol ie.
Whether it is primary secondary or tertiary and on the conditions. Partial Oxidation of Primary Alcohols Reaction. Primary alcohol aldehyde Reagent.
Potassium dichromate VI solution and dilute. Chemical Oxygen Demand COD results may differ when measured via the PeCOD COD method versus the traditional dichromate COD method for certain sample matrices. There are various reasons for this difference.
One is that chloride ammonia and some heavy metals have been known to interfere with PeCOD readings and provide inaccurate results. Another reason could be the time delays between. Every compound has its own CHEMICAL FORMULA and its own NAME.
The nomenclature naming systems for IONIC and MOLECULAR compounds are different. These consist of any positive ion except H combined with any negative ion. If H is the positive ion the compound is an acid as we will see later on page 6 The positive ion cation may be a monatomic metal ion such as.
2A solution of potassium chloride when mixed with silver nitrate solution an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction. It is a double displacement reaction.
It is also a precipitation reaction as AgCl is a white precipitate. 3Write the balanced chemical equations for the following reactions.