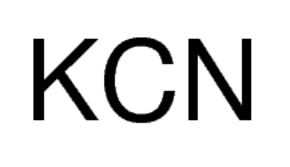
Must be in the context of the correct reagent 2. Ignore hard to digest easily digested 2.
This follows the trend observed in most.
Potassium cyanide ph level. The excretion of 14C-labeled cyanide in rats exposed to chronic intake of potassium cyanide was studied in rats exposed to daily intake of labeled potassium cyanide in the diet for 6 weeks. Urinary excretion was the main route of elimination of cyanide carbon in these rats accounting for 83 of the total excreted radioactivity in 12 hr and 89 of the total excreted radioactivity in 24 hr. Potassium cyanide decomposes on contact with water humidity carbon dioxide and acids producing very toxic and highly flammable hydrogen cyanide gas.
Potassium cyanide solution in water is a strong base. It reacts violently with acid and is corrosive. Potassium cyanide undergoes violent chemical reactions with chlorates and nitrites.
Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19.
Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash the ashes of plants from which its name derives.
53-87 5 soln Vapor Pressure. 1 hPa 20 deg C Vapor Density. No data Evaporation RateNegligible Viscosity.
No data Boiling Point. 500 deg C FreezingMelting Point170-179 deg C Decomposition TemperatureNot available. Specific GravityDensity1886 Molecular FormulaKSCN Molecular Weight9718 Section 10 - Stability.
Hydrogen cyanide sometimes called prussic acid is a chemical compound with the chemical formula HCN. It is a colorless extremely poisonous and flammable liquid that boils slightly above room temperature at 256 C 781 FHCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Melting together with an ammonium salt leads to a violent explosion Von Schwartz 1918 p.
A mixture with potassium cyanide may cause an explosion. When a little ammonium sulfate is added to fused potassium nitrite a vigorous reaction occurs attended by flame Mellor 2702. If the level of lead in a childs blood is at or above the CDC action level of 5 micrograms per deciliter it may be due to lead exposures from a combination of sources.
EPA estimates that drinking water can make up 20 percent or more of a persons total exposure to lead. Infants who consume mostly mixed formula can receive 40 percent to 60 percent of their exposure to lead from drinking water. Select when the highest level of respiratory protection is necessary but a lesser level of skin protection is required.
This is the minimum protection for workers in danger of exposure to unknown chemical hazards or levels above the IDLH or greater than AEGL-2. It differs from Level A in that it incorporates a non-encapsulating splash-protective chemical-resistant splash. HI-1144B pH Combination Electrode for Tris Buffer.
HI-1413B Glass Bodied Flat pH Electrode BNC Connector. HI-1414D50 Flat Tipped pH Electrode with short body - 50mm. HI-1617D Intelligent pH Electrode Spear Point for Emulsions.
Example potassium cyanide is a systemic toxicant in that it affects virtually every cell and organ in the body by interfering with the cells ability to utilize oxygen. Toxicants may also affect only specific tissues or organs while not producing damage to the body as a whole. These specific sites are known as the target organs or target tissues.
The main use of potassium chloride is in the manufacture of fertilizers. It finds use in medicine as an electrolyte replenisher and to treat hypokalemia with its pharmaceutical preparation a condition in which the level of potassium will be low in blood. Further it finds use as a flux agent in welding and metal casting.
It is useful in water softener units certain de-icing products and. Add iodine potassium iodide solution to the food sample. Must be in the context of the correct reagent 2.
Blue black purple indicates starch is present. Starch digested to maltose by amylase. Ignore hard to digest easily digested 2.
Maltose digested to glucose by maltase. Digestion of sucrose is a single step only one enzyme sucrase. Cyanide poisoning may result from inhalation ingestion or dermal exposure to various cyanide-containing compounds including smoke from closed-space fires.
Sources of cyanide poisoning include hydrogen cyanide and its salts cyanogenic plants aliphatic nitriles and prolonged exposure to sodium nitroprusside. The presence and extent of cyanide poisoning are often initially unknown. For example the two most extreme pH values at which the experiment was conducted pH 4 and pH 10 were also the values at which the paper disc took the most time to fall and rise 893s and 675s respectively.
This contrasts directly to the amount of time taken by the disc at the optimum pH of 7 where the average value was the much lower 451s. This follows the trend observed in most. Cyanide Titration of anionic and cationic surfactants including in chloroform phPotassium Electroplating metal processing Determination of Al Ba Bi Ca Cd Co Fe Mg Ni Pb Zn in accordance with AB 101 Water Titrations in ion-deficient aqueous media Paints lacquers solvents Fluoride Food semi-luxury items.
The pH values of different stages of titration shows that at first the pH changes very slowly and rise to only about 4. Further addition of such a small amount as 001 mL of the alkali raises the pH value by about 3 units to pH 7. Now the acid is completely neutralized.
Further of about 001 mL of 01 M NaOH will amount to adding hydrogen ions and the pH value will jump to about 9. Peracetic Acid PAA pH Phenols Phosphonates Phosphorus Potassium Q Quaternary Ammonium Compounds S Salinity Selenium Silica Silver Sodium Solids Nonfilterable Suspended Solids Total Solids Total Filterable Solids Total Volatile and Fixed Solids Volatile Dissolved Fixed Dissolved Sulfate Sulfide Hydrogen Sulfide Sulfite Sulfur Dioxide T. Our innovations include the first tester with a replaceable pH electrode the first combination testers that can measure multiple parameters the first with a large multi-level LCD that shows both pH and temperature readings and now to an extensive line of application specific testers that are optimally designed for difficult samples including cheese meat bread wine and beer to name a few.
At level 2 the pH meter will flash if any of the reactions are outside of the pH optimal. At level 3 the reaction chamber will be able to follow your reaction progress in real time. At level 4 the reaction chamber will be able to determine the reaction quality in real time displaying the effects of purity pH and other factors along a dial.
Solutions potassium gold cyanide containing at least sixty-eight and three-tenths percent 683 gold potassium silver cyanide containing at least sixty-eight percent 68 silver and silver cyanide in salt solution containing at least fifty-four percent 54 silver. Page 6 of 38 o. Precious stones refers to all gems and stones used in jewelry making such as gemstones jewels and.
Record accurate and reliable pH ion concentration mV ORP and temperature measurements with the Thermo Scientific Orion Star A214 pH and ISE Benchtop Meter. This meter is ideal for a wide range of applications and advanced pH or ion analysis in the lab. Perform up to 5 point pH and ISE calibration.
C ethanolic potassium cyanide D ethanolic sodium hydroxide 25 1-chloro-1-methylcyclohexane is hydrolysed by heating with NaOHaq. CH3 Cl OH CH3 OH Cl The reaction pathway is shown. Energy reaction pathway Z One carbon atom in 1-chloro-1.
Interfering substance Interference level Acidity If the sample is extremely acidic pH 2 or less a precipitate may form. Add 8 N Potassium Hydroxide Standard Solution by drops until the sample pH is above 4 then start the test. Aluminum Al 3 Reagents accommodate high levels.
Cyanide CN-Prevents full color development. Add 05 mL of. Acidity is the level of acid in substances.
Basicity refers to the state of being a base which can release hydroxide ions OH-. Acidity causes a low pH in aqueous mediums. Basicity causes a high pH in aqueous mediums.
Acidity indicates a high concentration of hydrogen ions in a medium.