
01 dextran 7003 methylcellulose 2910. 1 INDICATIONS Type 2 Diabetes Mellitus T2DM MonotherapyFORXIGA dapagliflozin propanediol monohydrate is indicated for use as an adjunct to diet and exercise to improveglycemic control in adultpatients with T2DM for whom metformin is inappropriate due to contraindications or intolerance.
Hydrogenated vegetable oil magnesium stearate polyethylene glycol polyvinyl alcohol silicon dioxide talc and titanium dioxide.
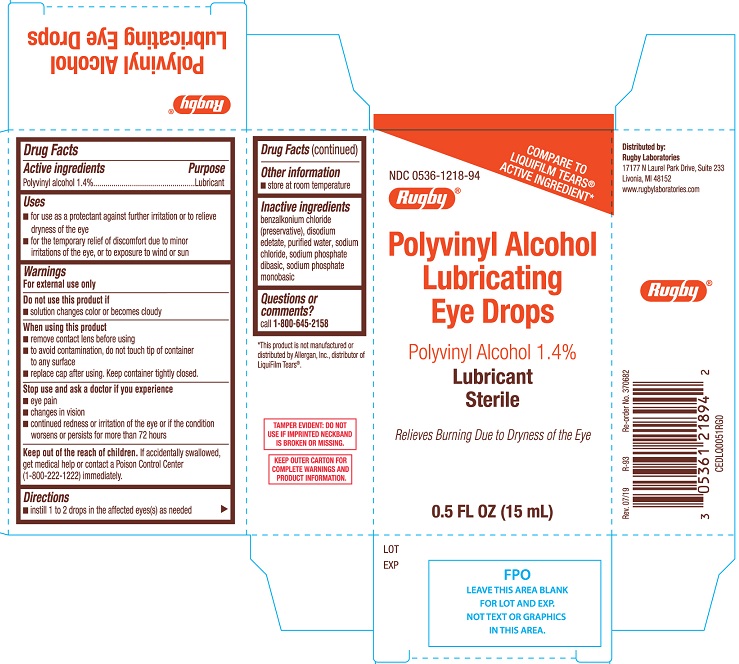
Polyvinyl alcohol indications. Treatment with Pristiq at all doses from 50 mg per day to 400 mg per day in controlled studies was associated with sustained hypertension defined as treatment-emergent supine diastolic blood pressure SDBP 90 mm Hg and 10 mm Hg above baseline for 3 consecutive on-therapy visits see Table 7Analyses of patients in Pristiq pre-marketing short-term controlled studies who met. Hemostatic agents include temporary embolic material such as gelatin sponges that degrade within days to weeks or more permanent devices such as. 1 polyvinyl alcohol1 polyethylene glycol 400.
04 polyethylene glycol 40003 propylene glycol. 01 dextran 7003 methylcellulose 2910. 01 dextran 7002 glycerin03 hydroxypropyl methylcellulose 2910.
Ophthalmic solution without preservative. 01 dextran 7003 hydroxypropyl methylcellulose 2910. 06 glycerin06 propylene glycol.
Polyethylene terephthalate PET is a chemically stable polyester and its use has risen dramatically in the last few decades with a multitude of applications ranging from food and drink containers to the manufacture of electronic components 176196 176 196 and as fibres in clothes. Often recycled PET bottles are used to make fleece garments as well as plastic bottles. Hydrogenated vegetable oil magnesium stearate polyethylene glycol polyvinyl alcohol silicon dioxide talc and titanium dioxide.
Yellow tablets also contain DC Yellow No. 10 aluminum lake and FDC Yellow No. Blue tablets also contain FDC Blue No.
1 aluminum lake and FDC Blue No. 3 pharmacies near 98837 have. The inactive ingredients of LINZESS 72 mcg capsules include.
Calcium chloride dihydrate L-histidine microcrystalline cellulose polyvinyl alcohol and talc. The components of the capsule shell include gelatin and titanium dioxide. Encapsulation of ginger Zingiber officinale Roscoe essential oil GEO in different amounts ie.
1 3 and 5 vv within chitosan nanoparticles CNPs was studied. It was done by a two-step method. Oil in water emulsion OW and ionic gelation using sodium tripolyphosphate STPP as the crosslinking agent.
GEO-loaded CNPs appeared to be more aggregated with an increase in the GEO. 1 INDICATIONS AND USAGE RADICAVA is indicated for the treatment of amyotrophic lateral sclerosis ALS. 2 DOSAGE AND ADMINISTRATION 21 Dosage Information The recommended dosage of RADICAVA is an intravenous infusion of 60 mg administered over a 60-minute period according to the following schedule.
An initial treatment cycle with daily dosing for 14 days followed by a 14-day drug. Amitriptyline hydrochloride USP is supplied as 10 50 75 100 and 150 mg tablets. The inactive ingredients are croscarmellose sodium magnesium stearate and silicified microcrystalline cellulose.
The tablet coating ingredients are polyvinyl alcohol polyethylene glycol talc and titanium dioxide. In addition the tablet coating also contains. Citric Acid Polyvinyl Alcohol Sodium Citrate.
Hydrochloric Acid andor Sodium Hydroxide may be added to adjust pH 56 to 66 and Water for Injection. Brimonidine Tartrate Ophthalmic Solution is an alpha adrenergic receptor agonist. It has a peak ocular hypotensive effect occurring at two hours post-dosing.
Fluorophotometric studies in. Use glass bottles and non-PVC polyvinyl chloride tubing to avoid adsorption of drug to delivery devices. Use of PVC tubing in infusion sets may lead to loss of active ingredient due to adsorption of nitroglycerin to PVC tubing.
Adsorption by PVC tubing is increased when tubing is long flow rates are low and nitroglycerin concentration of solution is high. Indications and Usage 1. 57 Benzyl Alcohol Toxicity in Pediatric Patients Gasping Syndrome 58 Risk Associated with Concurrent Use of Leucovorin for.
59 Folate Deficiency 510 Hemolysis. 511 Infusion Reactions 512 Hypoglycemia. 513 Impaired Phenylalanine Metabolism 514 Porphyria and Hypothyroidism.
515 Potential Risk in the Treatment. For example isopropyl alcohol has greater lipophilic properties than ethyl alcohol and is less active against hydrophilic viruses eg poliovirus. Generally the antimicrobial activity of alcohols is significantly lower at concentrations below 50 and is optimal in the 60 to 90 range.
Little is known about the specific mode of action of alcohols but based on the increased efficacy in. Artificial tears are lubricating eye drops used to relieve dryness and irritation of the ocular surface. Dry eye syndrome keratoconjunctivitis sicca is a common ocular surface disorder and is characterized by disruption of the tear film and increased inflammation.
The tear film coats the surface of the eye and is composed of 3 layers. An aqueous lipid and mucous layer. Another group developed drug loaded finasteride microspheres novel designed finasteridepoly 3-hydroxybutyrate-3-hydroxyvaleratepolyvinyl alcoholchitosan tested in an animal model.
This approach demonstrated prolonged release of the drug for up to 51 days with an excellent biocompatibility profile. In vivo studies performed on rabbit ears did demonstrate evidence of ischemia. In patients with cirrhosis secondary to alcohol abuse the mean total clearance of methocarbamol was reduced approximately 70 compared to a normal population 119 Lhr and the mean elimination half-life was extended to approximately 34 hours.
The fraction of methocarbamol bound to plasma proteins was decreased to approximately 40 to 45 compared to 46 to 50 in an age and weight-matched. INDICATIONS AND USAGE. BRIVIACT is indicated as adjunctive therapy in the treatment of partial-onset seizures in patients 16 years of age and older with epilepsy.
DOSAGE AND ADMINISTRATION Dosage Information When initiating treatment gradual dose escalation is not required. The recommended starting dosage is 50 mg twice daily 100 mg per day. Based on individual patient.
A Prospective Randomized Study Comparing Tris-acryl Gelatin Microspheres versus Polyvinyl Alcohol Microspheres. J Vasc Interv Radiol 2008. The primary embolic agent used was calibrated microspheres in 73 of cases either Embosphere or Embosphere Gold Biosphere Medical Rockland MA Siskin GP et al.
Leiomyoma Infarction after Uterine Artery Embolization. The main materials are alginates gelatin agar waxes thermoplastics metal oxides polyethylene glycol polyvinyl alcohol polyacrylate polystyrene and methacrylate. According to Brandau 2002 the process is based on pumping of the material to be microencapsulated through a nebulizer nozzle.
Inert ingredients in the 60mg nifedipine extended-release tablet formulation are lactose monohydrate microcrystalline cellulose hypromellose magnesium stearate polyvinyl alcohol titanium dioxide talc macrogolpolyethylene glycol 3350 lecithin soy iron oxide red iron oxide black and iron oxide yellow. The following infusion systems have been tested and found satisfactory. Unit-dose polyvinyl chloride and polyolefin containers.
No other systems have been tested and therefore no others can be recommended. Dilution EACH 5 ML OF SULFAMETHOXAZOLE AND TRIMETHOPRIM INJECTION SHOULD BE ADDED TO 125 ML OF 5 DEXTROSE IN WATER. 1 INDICATIONS Type 2 Diabetes Mellitus T2DM MonotherapyFORXIGA dapagliflozin propanediol monohydrate is indicated for use as an adjunct to diet and exercise to improveglycemic control in adultpatients with T2DM for whom metformin is inappropriate due to contraindications or intolerance.
Add-on combinationFORXIGA is indicated in adult patients with T2DM to improve glycemic control in. Reinforce indications and recommended practices for non-sterile disposable glove use. Prioritize sterile gloves for surgical and other sterile procedures.
Medical gloves for handling chemotherapy agents chemotherapy gloves should be prioritized for HCP handling chemotherapy and other hazardous drugs. Remind HCP about indications for when gloves are needed as well as. Refer Table 1 for composition and indications of use of various plasma products Table 1.
Various plasma products and their indications. Other human plasma derivatives. These include FEIBA factor VIII bypassing activity concentrate Antithrombin Fibrinogen Fibrin sealant FS Protein C C1 esterase inhibitor Blood products can be modified to make blood transfusion safer and accessible.