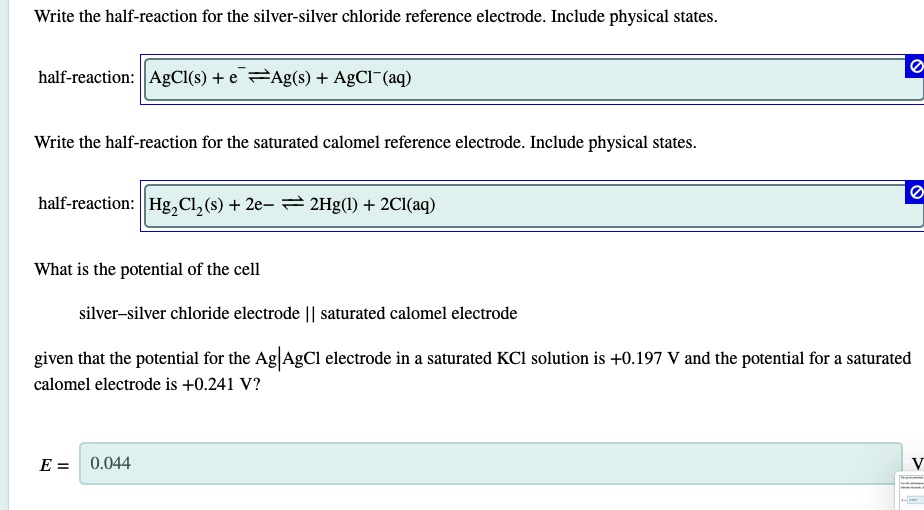
Unlike these three ionic compounds AgCl does not dissolve in water to a significant extent as signified by its physical state notation s. Silver chloride is a white crystalline chemical compound with the formula AgCl.
Hao Zheng Alexander Weismann and.
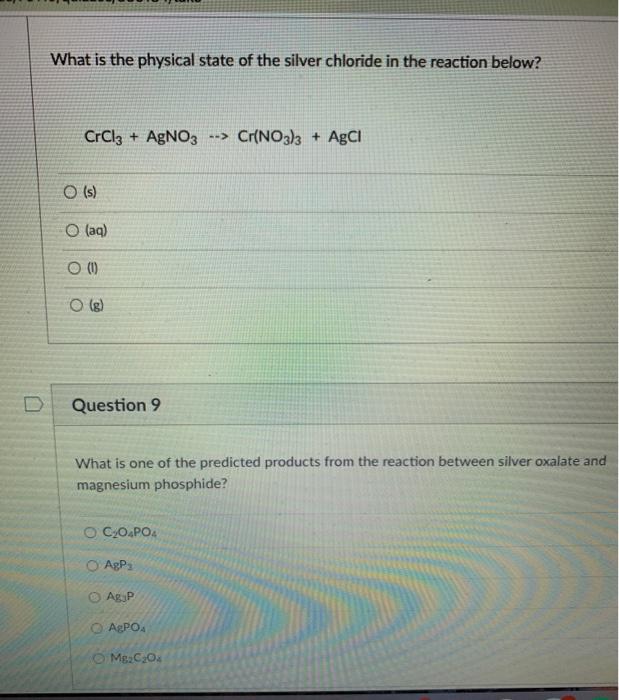
Physical state of agcl. Silver chloride is a white crystalline chemical compound with the formula AgCl. Silver chloride in the test tube quickly turns purplish especially in a sunny laboratory because the silver chloride is split up into silver and chlorine. Silver chloride is prepared when sodium chloride is added to silver nitrate solution a white precipitate of silver chloride occurs.
Silver chloride is an. Here we report a 60-nt DNA label-free detection strategy without pretreatment by SERS with polyquaternium-modified Ag microcrystals derived from an AgCl cube. Through the reduction-induced decomposition the size of the about 3 3 3 μm3 AgCl cube is reduced to Ag and the surface is distributed with the uniform size of 63 nm silver nanoparticles providing a large area of a robust and.
Standard Electrode Potential is the Potential that Arises in a Cell under Standard Conditions. Detailed Explanation of Standard Electrode Potential Provided Here. The LibreTexts libraries are Powered by MindTouch and are supported by the Department of Education Open Textbook Pilot Project the UC Davis Office of the Provost the UC Davis Library the California State University Affordable Learning Solutions Program and Merlot.
We also acknowledge previous National Science Foundation support under grant numbers 1246120 1525057 and 1413739. The Journal of Physical Chemistry C 2021. Observation of a Shockley Surface State on Gold Nanoparticles with Sizes Down to 5 nm.
Hao Zheng Alexander Weismann and. The Journal of Physical Chemistry C 2021 125 45 25327-25331 C. Physical Properties of Materials and Interfaces Publication Date Web.
After passing through a high energy transition state. For example the physical change in which water melts into a liquid may be represented by the equation. H 2 Os H 2 Ol The chemical formulas of the reactants and products are the same.
Examples of Products. Silver chloride AgCl s is the product of the reaction between the silver cation and chloride anion in aqueous solution. Essentials of Physical Chemistry by BS.
Essentials of Physical Chemistry by BS. Full PDF Package Download Full PDF Package. A short summary of this paper.
23 Full PDFs related to this paper. Essentials of Physical Chemistry by BS. During a physical science lab investigating chemical reactions several students placed a 30g antacid tablet in a 30g zip-lock bag.
They recorded the masses of the tablet and the bag. Then they added 50 grams of water and quickly sealed the bag. The tablet began to fizz and soon disappeared.
The bag was filled with gas. If the mass of the liquid after the reaction is completed is still 50. A commonly quoted example of this is silver chloride AgCl.
Depending on where you get your data from the theoretical value for lattice enthalpy for AgCl is anywhere from about 50 to 150 kJ mol-1 less than the value that comes from a Born-Haber cycle. In other words treating the AgCl as 100 ionic underestimates its lattice enthalpy by quite. Solubility Effects on Reactions.
Depending on the solubility of a solute there are three possible results. 1 if the solution has less solute than the maximum amount that it is able to dissolve its solubility it is a dilute solution. 2 if the amount of solute is exactly the same amount as its solubility it is saturated.
3 if there is more solute than is able to be dissolved the excess. Atkins Physical Chemistry 7th Solution Manual. Full PDF Package Download Full PDF Package.
A short summary of this paper. 20 Full PDFs related to this paper. Atkins Physical Chemistry 7th Solution Manual.
Electrospinning is a highly versatile method that can fabricate ultrafine fibers from various materials either in the form of individual fibers or nonwoven fiber mats with nanometer-to-micrometer size diameters which are 10 2 10 4 times smaller than those fabricated by the conventional approaches of solutionmelt spinning. Synthesizing fibers using electrostatic. The physical state of chemicals is also very commonly stated in parentheses after the chemical symbol especially for ionic reactions.
When stating physical state s denotes a solid l denotes a liquid g denotes a gas and aq denotes an aqueous solution. If the reaction requires energy it is indicated above the arrow. A capital Greek letter delta is put on the reaction arrow to show.
Vigouroux was the first researcher 4 to uncover a link between mental state and GSR activity finding an association with the level of sedation in patients and skin resistance 1 5. This connection of emotional response to GSR signal has been explored in thousands of articles in the 120 years since this seminal finding review article. While sweat secretion plays a major role for.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution therefore materials in other forms may need to be brought to this state before using standard methodsThe solution is then treated with various reagents to test for reactions. Also remember to state the exact time the writer should take to do your revision.
We offer free revision as long as the client does not change the instructions that had been previously given. In case a client want to alter the instructions revision can be done but at a negotiated fee. We give 100 refund for an assignment that we cant complete that had been paid for.
Will my paper be. Important Questions for Class 12 Chemistry Chapter 1 The Solid State Class 12 Important Questions The Solid State Class 12 Important Questions Very Short Answer Type Question 1. Which point defect in crystals does not alter the density of the relevant solid.
Which point defect in its crystal. Conductors in solid state as well as in molten state Physical Nature. It includes ZnSAgClAgBrand AgI.
Different types of non-stoichiometric defects. This defect arises because of absence of metal ions from its lattice sites. The electrical neutrality is maintained by an adjacention having a higher positive charge.
Reasons for the cause of metal. With a vast coverage of General Chemistry and Physical Chemistry the book exemplifies various topics including Basic concepts of chemistry Mole Concept Advanced Stoichiometry Atomic structure Nuclear Chemistry Chemical Bonding Ideal Gas Equation Kinetic Theory of Gases Euidiometry Non-ideal Gases The liquid state and Thermodynamics. Quite a few crucial topics like Atomic Structure.
The term reference state generally refers to elements and is the thermodynamically most stable state of the element at the temperature of interest. The distinction between standard state and reference state for elements may seem slight but becomes clear for those elements that can exist in more than one form at a specified temperature. Physical chemistry of foams and aerosols.
The educational manual consists of a theoretical part and an experimental part. The manual presents the laboratory works. 295 26 1MB Read more.
Physical chemistry for mathematicians in tasks and questions. Educational manual 9786010415706. Textbook is of physical chemistry is designed for students mathematical.
Factors such as climate electrolyte conductivity and chemistry physical loading and biological activity all impact CP requirements. These factors are generally allowed for in the CP current demand and polarization potential criteria. 4 40 CP CRITERIA Potential measurements are the most commonly used criteria to ascertain the level of CP afforded to metals and alloys.
CP potential values. 13 Physical and Chemical Properties External Url 13 Physical and Chemical Properties 13 Physical and Chemical Properties 5 external_url 1183109 0. A state-of-the-art scenario analysis shows that implementing the most aggressive solutions from 2016 to 2040 through the use of currently available knowledge and technologies including collection disposition recycling reduction and substitution could reduce plastic pollution by 40 relative to 2016 but 710 Mts of plastic waste will still be discharged inescapably into aquatic and.
Unlike these three ionic compounds AgCl does not dissolve in water to a significant extent as signified by its physical state notation s. Explicitly representing all dissolved ions results in a complete ionic equation. In this particular case the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions.
Ca 2 a q 2 Cl a q 2 Ag a q 2.