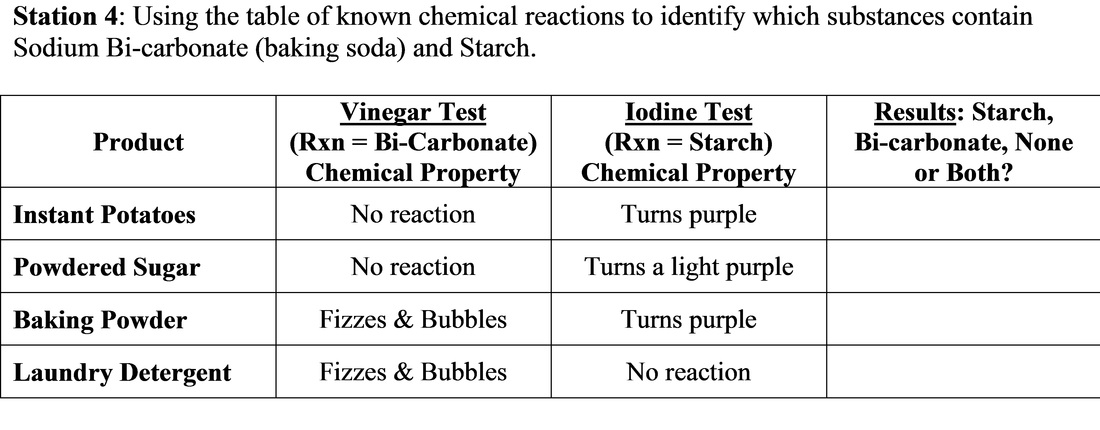
The reaction between baking soda and mildly acidic household substances such as vinegar or orange juice form the basis for several kitchen science experiments such as the volcano. Properties of Sodium Bicarbonate.
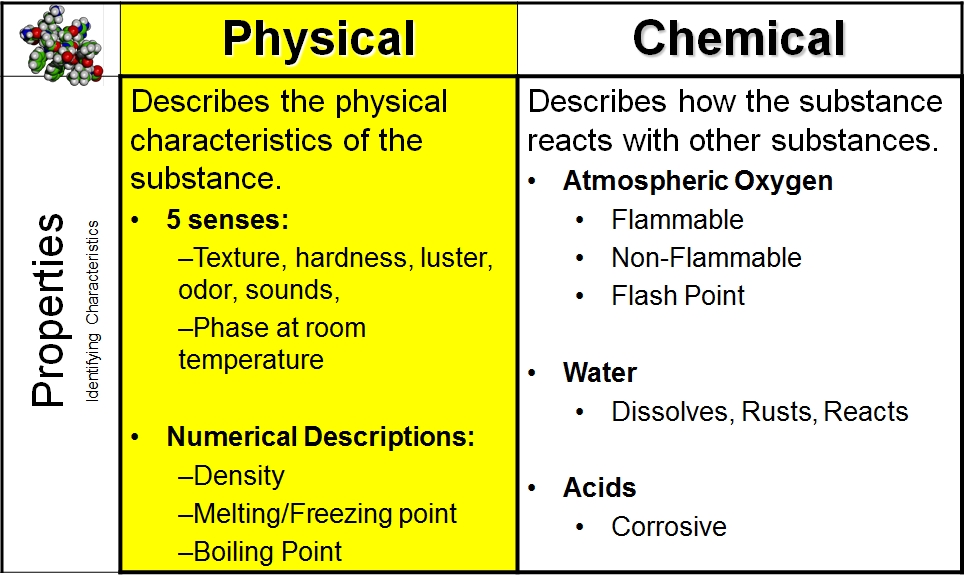
Identify the following as a chemical C or physical property P.
Physical properties of baking soda. Some of the physical and chemical properties of sodium bicarbonate have useful applications. Baking sodas alkalinity causes it to react with acids. This property makes sodium bicarbonate useful for baking cleaning and deodorizing.
Many bad odors are caused by acids and baking soda neutralizes these odors when it reacts with them. The gas released during an acid-base. The important chemical reaction that is used in the production of baking soda and sodium carbonate is.
CO 2 H 2 O NH 3 NaCl NaHCO 3 NH 4 Cl 2 NaHCO 3 Na 2 CO 3 CO 2 H 2 O. Carbon dioxide produced is recycled to produce NaHCO 3. Properties of Sodium Bicarbonate.
Powder dust is not as explosive. It has a. Baking soda can even be used in the conservation of old or fragile paper with a high acid content where it can act as a neutralizer and buffer against further decay.
New research is into the science of baking soda is focusing on larger-scale applications of baking sodas cleaning and absorbent properties. Baking soda has antibacterial and antimicrobial properties. One of the reasons why baking soda is good for your health is that it contains antibacterial and antimicrobial properties that can help reduce infections.
When discussing the antimicrobial activity of sodium bicarbonate the Journal of Food Science found that baking soda can kill off various strains of bacteria for example. Baking soda also known as sodium bicarbonate or bicarbonate of soda is a popular baking ingredient. It gives foods like bread cakes muffins and cookies a light fluffy texture.
It is the natural chemical and physical properties of Baking Soda that account for its many safe and effective uses. The five specific capabilities of Baking Soda are listed below. Baking Soda acts a cleaning agent because it is a mild alkali and can cause dirt and grease to dissolve easily in water for effective removal.
When it is not fully dissolved like when it is sprinkled on a. Much like a salt or sugar scrub baking soda acts as a physical exfoliator when made into a paste or not fully dissolved in water. Exfoliating can be useful but exfoliating day and night like.
Sodium bicarbonate NaHCO3 or CHNaO3 CID 516892 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Baking soda helps to regulate the pH level in the washers water by keeping it from being too acidic or alkaline.
By adding 12 cup of baking soda to each laundry load detergents can work more effectively and reduce bacteria. For heavy odor problems like underarm perspiration or environmental odors like cigarette smoke use baking soda and water as a pre-soak. Dissolve 1 or 2 cups of baking.
An example of this would be adding baking soda and vinegar and watching it bubble and give o a gas. The bubbling is an indicator that the properties of the two initial ingredients have recombined to form a new substance or substances. Substanc e AB substance CD new substanc e AD new substance BC A simple equa tion of what happens when you add baking soda to vinegar.
Ing soda solid. Baking soda is converted to a baking powder by adding tartaric acid and starch to the baking soda. Baking soda is basic in nature and bitter in flavor.
As the baking soda reacts to the batter that is produced it neutralizes the bitter taste of the baking powder to. Upon completion the learner will be able to differentiate between physical and chemical changes. Physical changes occur when objects undergo a change that does not change their chemical nature.
A physical change involves a change in physical properties. Physical properties can be observed without changing the type of matter. Mixing vinegar and baking soda initiates a chemical reaction that produces carbon dioxide and water.
The chemical names of the two ingredients are acetic acid which is vinegar and sodium bicarbonate which is baking soda. Don t go overboard with baking soda due to its alkaline properties too much of it can leave the area dry. Here are six fun facts about the vagina that you should know.
Use as bathing salt. Baking soda and vinegar react chemically because one is a base and the other is an acid. Baking soda is a basic compound called sodium bicarbonate.
Vinegar is a diluted solution that contains acetic acid. The baking soda and vinegar reaction is actually two separate reactions. The first reaction is the acid-base reaction.
The reaction between baking soda and mildly acidic household substances such as vinegar or orange juice form the basis for several kitchen science experiments such as the volcano. You put baking soda in a small empty plastic tube or soda bottle then add vinegar or fruit juice. The carbon dioxide makes the solution bubble and foam vigorously overflowing from the bottle.
PHYSICAL AND CHEMICAL. PROPERTIES AND CHANGES PHYSICAL PROPERTY CHEMICAL PROPERTY. Observed with senses 1.
Indicates how a substance. Determined without changing matter reacts with something else 2. Matter will be changed into a new substance after the reaction.
Identify the following as a chemical C or physical property P. Blue color P 8. Baking soda is also known to offer various health benefits when taken on its own.
Research suggests that using baking soda may help prevent the loss of. When citric acid and baking soda mix carbon dioxide is produced and the temperature decreases. This must be a chemical change.
Identify each of the following as a physical or chemical change. _____ You leave your bicycle out in the rain and it rusts. _____ A sugar cube dissolves.
_____ Scientist break-up water into oxygen and hydrogen gas. _____ Burning coal for a barbecue. Burning wood is a physical change.
Combining hydrogen and oxygen to make water is a physical change. Breaking up concrete is a physical change. Sand being washed out to sea from the beach is a chemical change.
When ice cream melts a chemical change occurs. Acid rain damaging a marble statue is a physical change. Hi lots of people know a few things that Baking Soda can do another one that most people have never heard of was suggested to me about 5 years ago before my retirement I was a university history professor and at that time I had 3 80 minute classes per day 2 in the morning and 1 in the afternoon unfortunately they were all at the same level and were learning the same knowledge and I.
A rock is an aggregate of one or more minerals or mineraloids. Some rocks such as limestone or quartzite are composed primarily of one mineral calcite or aragonite in the case of limestone and quartz in the latter case. Other rocks can be defined by relative abundances of key essential minerals.
A granite is defined by proportions of quartz alkali feldspar and plagioclase feldspar. A change in the physical and chemical properties 2. A new substance IS formed Identify the following as physical P or chemical C changes.
Part A NaCl Table Salt dissolves in water Ag Silver tarnishes. An apple is cut. Heat changes to steam.
Baking soda reacts to vinger. Alcohol evaporates Ice melts. Because baking soda is a natural anti-inflammatory baking soda appears to direct immune cells to reduce inflammation instead of prompting it.
In other words baking soda helps boost the bodys anti-inflammatory response putting out a calming signal instead of an emergency attack signal which is most likely why baking soda is effective at helping colds flu and sore throats. When you look at baking soda vinegar drain cleaning instructions youll notice that they recommend following the baking sodavinegar. Soda has worked for drains specifically and it has helped for me it is only if you stopper the drain so that the physical not chemical force of the reaction helps to physically force some of the gunk out of the pipes.
That only works if you use a.