Nizkorodov Environmental Science Technology 2021 55 21 14586-14594 Anthropogenic Impacts on the Atmosphere Publication Date Web. The excited intermediate II is a strong oxidant which can generate radicals upon single-electron transfer SET activation of easy-to-oxidize substrates including organic silanes.
TAS reduced glutathione GSH.
Photochemical oxidant examples. Examples of photochemical reactions. Plants use solar energy to convert carbon dioxide and water into glucose and oxygen. The resulting singlet oxygen is an aggressive oxidant capable of converting C-H bonds into C-OH groups.
Photoresist technology used in the production of microelectronic components. Vision is initiated by a photochemical reaction. A solar fuel is a synthetic chemical fuel produced from solar energy.
Solar fuels can be produced through photochemical ie. Activation of certain chemical reactions by photons photobiological ie artificial photosynthesis thermochemical ie through the use of solar heat supplied by concentrated solar thermal energy to drive a chemical reaction and electrochemical reactions ie. We present here a review of the photochemical and electrochemical applications of multi-site proton-coupled electron transfer MS-PCET in organic synthesis.
MS-PCETs are redox mechanisms in which both an electron and a proton are exchanged together often in a concerted elementary step. As such MS-PCET can function as a non-classical mechanism for homolytic bond activation providing. _____ isare examples of secondary pollutants.
Volatile organic compounds C. Fugitive emissions are produced by A. Power plants and other heating equipment.
Reactions between pollutants and atmospheric gases. Strip mining rock crushing and other dust-producing activities. Photostability studies of drugs and drug products are an integral part of the product development process in the pharmaceutical industry.
These studies are carried out to ensure quality efficacy and safety of the formulated products during manufacture storage and use. This review deals with the concept of photostability and related aspects and the literature available in the field. The excited intermediate II is a strong oxidant which can generate radicals upon single-electron transfer SET activation of easy-to-oxidize substrates including organic silanes.
12a If this photochemical SET mechanism could be used to activate allyl silanes 2 too then an allylic radical I would be generated. A stereocontrolled radical coupling between I and the chiral 5π-electron β. The most relevant examples discussed above can be highlighted by for instance the email protected 2 Bi 2 MoO 6 copperII β-tetranitrophthalocyanine deposited on the surface of semiconducting CeO 2 Bi 2 MoO 6 nanoflowers which when used in 15 gL concentration showed high catalytic performance in the photodegradation of antibiotic tetracycline 005 gL concentration with.
Good examples are the retinoids including the visual pigments and their models. All-trans - and 11-cis-retinal are of course the key chromophores in vision and laser flash photolysis has been used extensively to investigate the production of triplet states and 1 O 2 from these pigments see for a recent review. Furthermore retinol and other retinoids are important in the cosmetic.
1971 and carbon dioxide and photochemical oxidant emissions limits in 1973. As a result atmospheric and water pollution levels were improved significantly in a relatively short period of time. 1-3-4 Pollution Control Measures Lose Momentum Increased Awareness of.
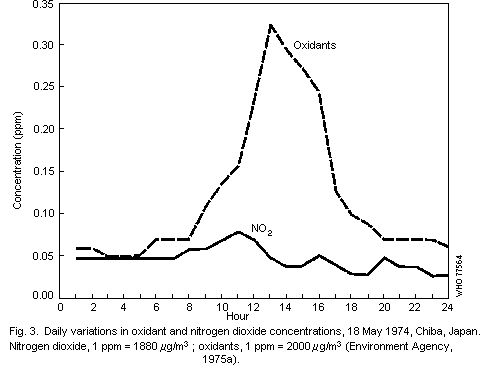
Contaminant of Concern. The term smog is a shorthand combination of smoke-fog However it is really the code word for photochemical oxidant smog the brown haze that can be seen when flying into Los Angeles St. Louis Denver and other metropolitan areas around the world.
Smog is made up of at least three ingredients. Light hydrocarbons and radical. How direct or indirect photochemical reactions involving ROS or organic radicals oxidize the DOM to form these three different classes of photoproducts each with important roles in C cycling Fig.
5 is poorly known in part because of the difficulty of isolating a specific oxidant and describing its. In this Account we highlight two recent examples from our group outlining the use of concerted PCET as a platform for the development of catalytic and enantioselective reactions of neutral ketyl radicals. Central to this work was the recognition that PCET provides a mechanism for independent proton and electron donors to function jointly as a formal hydrogen atom donor competent to activate.
Examples of these include Gombergs triphenylmethyl radical Fremys salt. It is gaining prominence as a model oxidant in small molecule and proteintherapeutics for its ability to initiate oxidation reactions via both nucleophilic and free radical mechanisms. Measurement of the decrease in the amount of antioxidants eg.
TAS reduced glutathione GSH. Examples of Environmental Significance Statements. Below are some examples of Environmental Significance Statements which may help to guide you when preparing your submission.
Insights into HONO sources from observations during a solar eclipse. Nitrous acid HONO is a major often the dominant boundary layer precursor to the key daytime atmospheric oxidant OH. When the solution of barium chloride reacts with the solution of sodium sulphate white precipitate of barium sulphate is formed along with sodium chloride.
BaCl 2 aq Na 2 SO 4 aq BaSO 4 s Precipitate 2NaClaq When sodium hydroxide a base reacts with hydrochloric acid sodium chloride and water are formed. NaOHaq HClaq NaClaq H 2 Ol. Ozone is produced via a complex suite of photochemical reactions between nitrogen oxides NOx and hydrocarbons in sunlight.
High ambient O 3 caused massive dieback of mixed conifer forests specifically sensitive ponderosa and Jeffrey pines in the San Bernardino Mountains 9 10 and western slopes of the Sierra Nevada Mountains 11 in the 1960s and 1970s. Although ozone damage directly. In these examples.
Which hinges on the photochemical performance of the amido Cui complex and is therefore highly dependent on the N-nucleophile structure activates the alkyl halide by. Early examples of CH activation involved the stoichiometric use of high-valent transition metals such as platinum 235 or palladium 236 for total synthesis Fig. Directing groups inherent.
Some illustrative examples of novel synthetic methodologies including organocatalytic processes asymmetric syntheses or phase-transfer catalytic methods for the enantioselective incorporation of fluorine atoms into versatile molecular entities are covered in the current review focusing on main contributions of the last decade. A series of examples in. Still a third problem is the control of oxidant exportimport across international boundaries that is the coupling between regional and global scales.
We address the three categories in order. Oxidation Pathways in the Troposphere. Establish the sources photochemical transformations meteorological control and deposition of trace oxidants notably OH.
Photochemical Degradation of 4-Nitrocatechol and 24-Dinitrophenol in a Sugar-Glass Secondary Organic Aerosol Surrogate. Nizkorodov Environmental Science Technology 2021 55 21 14586-14594 Anthropogenic Impacts on the Atmosphere Publication Date Web. Synthesis and Hydrolysis of Atmospherically.
Determine the major organic product for the following reaction scheme chegg. 11 Examples of LCA applications according to ISO 140. Environmental packing regulation beverage packing 54.
Umwelbundesamt UBA 52 ISO 1997. Explain the following terms with suitable examples. A Oxidation b Reduction Answer.
A Oxidation is a process of addition of oxygen to a substance or removal of hydrogen from a substance for example Copper is oxidised to CuO as oxygen is added to copper. B It is the process of removal of oxygen from a substance or addition of hydrogen to a substance for example Copper oxide is. One of the first examples of this chemistry involved the arylation of benzylic and allylic CH bonds with cyanoaromatics in the presence of a photoredox catalyst and a thiol methyl thioglycolate and triisopropylsilanethiol proved to be optimal choices.
73 74 An interesting example is reported in Scheme 27 and involves the arylation of 25-dihydrofuran 104 by 14-dicyanobenzene 105.