Its main use is as a precursor to polymers. CaSigma-Aldrich offers a number of 14-Butanediol products.

α-Tertiary Propargylamine Synthesis via KA 2-Type Coupling Reactions under Solvent-Free Cu I-Zeolite Catalysis.
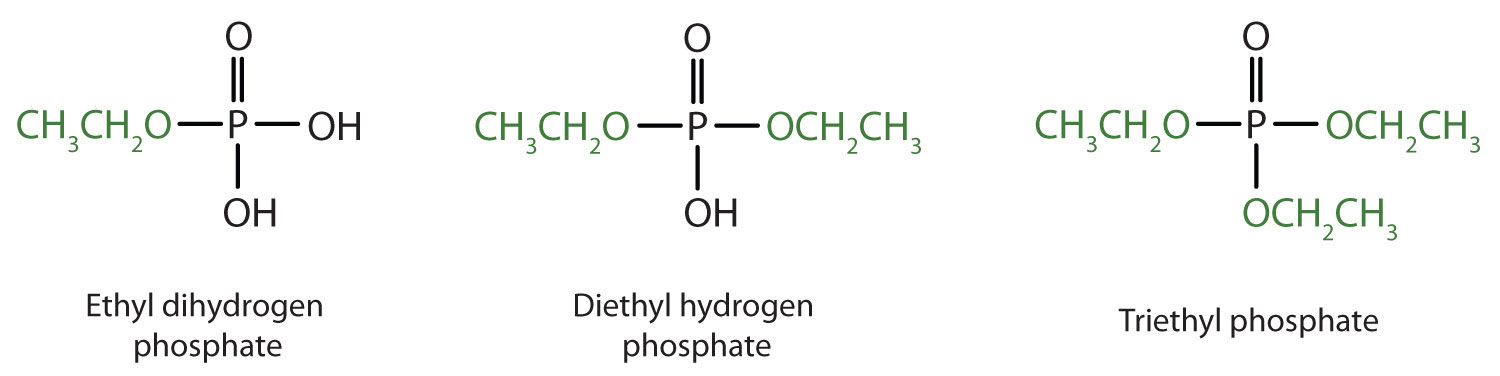
Phosphoric acid reactions with diols. Data for 2015 J S Plotkin The Propylene Quandary American Chemical Society 2016 2. Estimated data for 2014 Global Petrochemical Overview. Changing Olefins Markets Nexant and ChemVision 2014.
Sulfuric acid and perchloric acid are much stronger acids than the hydrogen acids HCl HBr HI which explains why sulfuric acid is commonly used to make olefins from alcohols. Reactions of n-butene and butan-2-ol in dilute acid. The elucidation of the mechanism and the intermediate in elimination from secondary alcohols and in the hydration.
Major industrial use is the production of phosphoric acid tert-Butyl hydroperoxide. Used in a variety of oxidation processes. Industrially is used as a starter of radical polymerization.
One of the most polar ethers. Its main use is as a precursor to polymers. The acidity of solutions of boric acid is known to increases with polyols containing cis-vicinal diols like mannitol and glycerol.
The value of pK of BOH3 is known to extend to five orders of magnitude from 9 to 4 under different concentrations of mannitol. It can be noted that in the presence of mannitol the solution of boric acid with the increased acidity can be referred to as. The reaction is catalyzed by phosphoric acid or sulfuric acid.
Reactions of the excited sensitizer can involve electron or hydrogen transfer usually with a reducing substrate Type I reaction or interaction with oxygen Type II reaction. These various alternative processes and reactions can be controlled by choice of specific reaction conditions leading to a wide range of products. Dehydration of alcohols requires a strong acid and is carried out at high temperatures 100-200 o C.
The most common strong acid used for dehydration is the concentrated sulfuric acid even though phosphoric acid and p-toluenesulfonic acid abbreviated as TsOH are often used as well. The reaction can follow both E1 and E2 mechanisms depending on whether it is a primary secondary or a. Inorganic esters also exist for instance esters of phosphorous acid H 3 PO 3 and phosphoric acid H 3 PO 4.
When starch granules are reacted with phosphorylating agents such as phosphoric acid mono- or di-starch phosphate is formed. The resulting starch has increased stability at high and low temperatures more resistant against acidic. Polyurethanes are made by the exothermic reactions between alcohols with two or more reactive hydroxyl -OH groups per molecule diols triols polyols and isocyanates that have more than one reactive isocyanate group -NCO per molecule diisocyanates polyisocyanates.
For example a diisocyanate reacts with a diol. The group formed by the reaction between the two molecules is known. A little acid can accelerate a wide range of chemical reactions.
The advent of chiral phosphoric acid derivatives has been useful for biasing these reactions toward just one of two mirror-image products. For the most part though these chiral catalysts have interacted with basic sites such as carbonyl groups. Now extend asymmetric acid catalysis to simple carbon-carbon double.
The most common interaction is with cis-12- or 13-diols of saccharides to form five- or six-membered rings respectively. The oxidation potential of the Fc became more anodic on binding with saccharides. 76 The interaction of the boronic acid and neighboring amine strengthened on saccharide binding thereby reducing the electron density on the neighboring amine.
Academiaedu is a platform for academics to share research papers. Not to worry. Search thousands of other internships scholarships and other student programs in 120 countries.
Search Or if you are wondering who we are. α-Tertiary Propargylamine Synthesis via KA 2-Type Coupling Reactions under Solvent-Free Cu I-Zeolite Catalysis. Fabian Schlimpen Clotilde Plaçais Eliot Starck Valérie Bénéteau Patrick Pale and.
Stefan Chassaing The Journal of Organic Chemistry Articles ASAP Article Publication Date Web. Ch3co2 name - restaurant-mandarinde. Chu Gong Yutao Zhao Dongmei Zhang Jie Wang Chaonan Mu Wei Wang Shoufei Zhu Xinxing Zhang Investigation of the Acid-Mediated Photosensitized Reactions of Amphiphilic αKeto Acids at the AirWater Interface Using FieldInduced Droplet Ionization.
Liang-Liang Yang Jing Ouyang Hui-Na Zou Shou-Fei Zhu and Qi-Lin. Ball Bulk Citric acid goes for 6148 oz 420 g or 1. CaSigma-Aldrich offers a number of 14-Butanediol products.
14 Butanediol is one of four stable isomers of butanediol. I kept redosing hoping the effects would get stronger but they do not all that happens is the euphoria stays the same and you get more and more tired. 4 Glycol Butyl 1 4-Butanediol price comparison get.
Toluene diisocyanate TDI is polymerized with diols to produce polyurethanes which are used to make flexible foam for furniture cushions mattresses and carpet pads. Trinitrotoluene TNT is made via a stepwise nitration of toluene in the 2 4 and 6 positionsTNT is a high explosive and missile propellant. Phthalic anhydride is made by air oxidation of.
Chiral Spiro Phosphoric Acid-Catalyzed Friedel-Crafts Conjugate Addition Enantioselective Protonation Reactions. Xue-Song Gu Na Yu Xiao-Hui Yang An-Te Zhu Jian-Hua Xie and Qi-Lin Zhou. Enantioselective Hydrogenation of Racemic α-Arylamino Lactones to Chiral Amino Diols with Site-Specifically Modified Chiral Spiro Iridium Catalysts.
Remdesivir is a nucleoside analog used to inhibit the action of RNA polymerase. 3 The duration of action is moderate as it is given once daily. 16 Due to much higher selectivity of mammalian DNA and RNA polymerases including human mitochondrial RNA polymerase for ATP over remdesivir triphosphate remdesivir is not a significant inhibitor of these enzymes which contributes to its overall.
Ch3co2 name - cdhmuniquspl. Ketone 20 also undergoes secondary reactions. Protection of the vicinal diols was carried out next using 22-dimethoxypropane catalyzed by 5 mol sulfuric acid in acetonitrile.
After completion of the reaction the MeOH produced from acetonide formation was removed by azeotropic distillation and then isobutyric acid anhydride triethylamine and catalytic DMAP 25 were added. Academiaedu is a platform for academics to share research papers. AAT Using Bioethanol as A Raw Material To Produce.
A Low Cost Nematicide And Fungicide Based on Ethyl Formate Which Is Generally Regarded as Safe by the FDA and Which Biodegrades Into Two Naturally Occurring Substances with No Lasting Detrimental Effects to Air Soil and Water. The Idea of Equivalents Concept Building Exercise 81 Concept Testing Exercise 81 Equivalent Mass in Acid Base Reactions Concept Building Exercise 82 Concept Testing Exercise 82 Equivalent Mass in a Redox Reaction Concept Building Exercise 83 Concept Testing Exercise 83 Exercise 1 Exercise 2 Chapter 9. Advanced Stoichiometry-I Acid Base and Precipitation Titrations Law of Conservation.