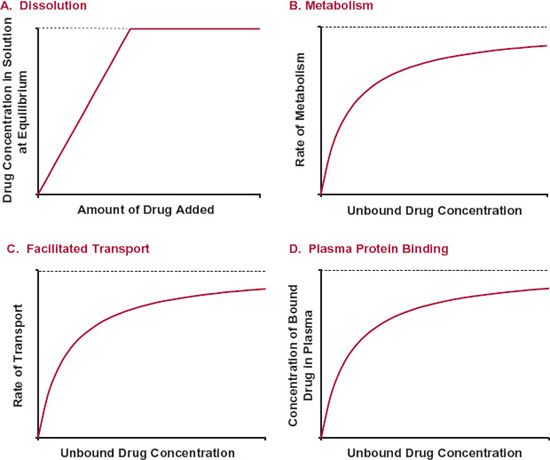
Links 138. In particular if the absorption and elimination systems are not saturable for small dosing increments the increase in.
Lesser extent due to saturable plasma protein binding.
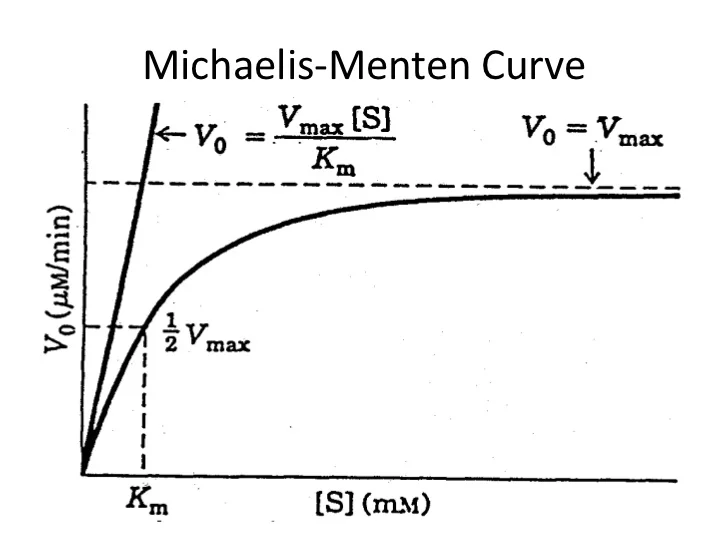
Phenytoin saturable kinetics. As opposed to linear kinetic drugs like vancomycin and aminoglycosides phenytoin exhibits non-linear kinetics. Meaning a change in dose does NOT produce a proportional change in concentration. Its also known as zero-order or saturable kinetics because metabolic mechanisms can literally become saturated.
This causes a constant AMOUNT of drug to be eliminated over time rather. Phenytoin kinetics All other drugs. The bodys capacity to metabolize phenytoin is fixed and saturable.
This is referred to as zero-order or Michaelis-Menten kinetics. The mnemonic Peas WHEATS has been used to remember all the drugs that have saturable metabolism. Phenytoin Phenylbutazone Warfarin Heparin Ethanol Acetaminophen Theophylline Tolbutamide Salicylates.
Because phenytoin exhibits saturable zero-order or dose-dependent pharmacokinetics the apparent half-life of phenytoin changes with dose and serum concentrations. This is due to the saturation of the enzyme system responsible for metabolizing phenytoin which occurs at therapeutic concentrations of the drug. Thus a constant amount of drug is metabolized capacity-limited metabolism and.
Elimination mechanisms are NOT saturable. A constant amount of drug is eliminated per unit time. For example 10mg of a drug maybe eliminated per hour this rate of elimination is constant and is independent of the total drug concentration in the plasma.
Zero order kinetics are rare Elimination mechanisms are saturable. Phenytoin is the classical poster child for non-linear elimination kinetics because the enzyme saturation point is reached somewhere in the middle of the therapeutic concentration range. The excellent 1979 article by Alan Richards contains within it a famous graphic representation of the changes in phenytoin concentration among a group of epileptic patients.
Since phenytoin obeys saturation kinetics the half-life is dependent on the plasma level. Plasma half-life is between 20 and 60 hours. It is normally shorter in children.
A prolonged half-life can be expected in premature and newborn babies as well as with toxic dosages. The therapeutic range for plasma concentration is generally between 10 and 20 µgml. Concentrations above 25 µgml may.
Aspirin pharmacokinetic parameters. Oral bioavailability F 68. Clearance CL 39 Lh.
Volume of Distribution Vd 105 L. Half-life t12 025 h. Salicylate pharmacokinetic parameters.
Clearance CL 36 Lh may decrease to 06 Lh depending on dose Volume of Distribution Vd 119 L. Half-life t12 2h may. Zero order kinetics then apply rather than the usual first order kinetics where a constant proportionof the drug in the body is eliminated per unit time.
Some examples of drugs which exhibit non-linear kinetic behaviour are phenytoin ethanol salicylate and in some individuals theophylline. Phenytoin exhibits marked saturation of metabolism at concentrations in the therapeutic. First-order and zero-order kinetics.
A fundamental aspect that requires attention is to evaluate the plasma concentrations of drugs after multiple administrations relate to the not-saturable and saturable mechanisms. In clinical terms the clinical differences are striking. In particular if the absorption and elimination systems are not saturable for small dosing increments the increase in.
Lesser extent due to saturable plasma protein binding. The kinetics of unbound drug are linear. Elimination Mean plasma clearance and volume of distribution for total valproate are 056 Lhr173 m2 and 11 L173 m2 respectively.
Mean terminal half-life for valproate monotherapy after an intravenous infusion of 1000 mg was 16 30 hours. Because the kinetics of unbound valproate are linear bioequivalence between DEPACON and DEPAKOTE up to the maximum recommended dose of 60 mgkgday can be assumed. The AUC and Cmax resulting from administration of IV valproate 500 mg as a single one hour infusion and a single 500 mg dose of DEPAKENE syrup to 17 healthy male volunteers were also equivalent.
Valproate displaces phenytoin from its plasma albumin binding sites and inhibits its hepatic metabolism. Co-administration of valproate 400 mg TID with phenytoin 250 mg in normal volunteers n 7 was associated with a 60 increase in the free fraction of phenytoin. Total plasma clearance and apparent volume of distribution of phenytoin increased 30 in the presence of.
Paroxetine does not alter the in vitro protein binding of phenytoin or warfarin. Again reflecting a saturable metabolic pathway. In comparison to.
Values after 20 mg daily values after 40 mg daily were only about 2 to 3 times greater than doubled. Paroxetine is extensively metabolized after oral administration. The principal metabolites are polar and.
Analysis of data by Michaelis-Menten kinetics yielded the propylene glycol metabolising rate as 063 gkgh. Propylene glycol metabolism saturation seems to occur at 29 gkg. Of note no CNS side effects were detected in rats given 29 gkg for up to 45 consecutive days.
These data are consistent with those of Yu et al. 122 following repeated oral doses in adult patients of either. Alcohol sometimes referred to by the chemical name ethanol is a psychoactive drug that is the active ingredient in drinks such as beer wine and distilled spirits hard liquor.
It is one of the oldest and most common recreational substances causing the characteristic effects of alcohol intoxication drunkenness. Among other effects alcohol produces happiness and euphoria decreased. Mcq on pharmacology 1.
All of the following are general mechanisms of drug permeation Except a Aqueous diffusion b Aqueous hydrolysis c Lipid diffusion d Pinocytosis or endocytosis e Special carrier transport 2. If the plasma concentration of a drug declines with first-order kinetics this means that a There is only one metabolic path for drug disposition b The half-life is. The release rate should follow zero order kinetics Kr rate in rate out KeVdCd Where Ke overall elimination first order kinetics.
Vd total volume of distribution. Cd desired drug concentration. B Dose consideration - To achieve the therapeutic level sustain for a given period of time for the dosage form generally consist of 2 part a Initial primary dose b maintenance.
This results in disproportionate increases in plasma concentrations of paroxetine and hence pharmacokinetic parameters are not constant resulting in non-linear kinetics. These properties are a consequence of the fact that one of the enzymes that metabolises paroxetine is the readily saturable cytochrome P450 enzyme 2D6 CYP2D6. However because this enzyme becomes saturated early on.
The first factor is the change in gastric pH. The majority of drugs orally administered requires to be dissolved and absorbed a gastric pH between 25 and 3. Therefore drugs able to increase gastric pH ie antacids anticholinergics proton pump inhibitors PPI or H2-antagonists can change the kinetics of other co-administered drugs.
Phenytoin and other anticonvulsant. Hadda Lester M Winchester James F. Links 138.
Luef G Burtscher J Kremser C Birbamer G Aichner F Bauer G et al. Magnetic resonance volumetry of the cerebellum in epileptic patients after phenytoin overdosages. Patterson Division of Infectious Diseases San Antonio Center for Medical Mycology The University of Texas Health Science Center at San Antonio 7703 Floyd Curl DriveMSC 7881 San Antonio TX 78229-3900 pattersonuthscsaedu.
Dict_fileseng_comdic This class can parse analyze words and interprets sentences. It takes an English sentence and breaks it into words to determine if it is a phrase or a clause. It can also counts the total number of words in a sentence checks if a word is a palindrome and can generate a new sentence with almost the same meaning using synonyms and other.