
Ulcerations of the mouth vomiting of blood and rapid appearance of shock convulsions twitching tetany and cardiovascular collapse may occur following ingestion of oxalic acid or its soluble salts. Oxalic acid in solution was given to the animals by gavage either with water alone or with 0625 gkg body wt of xylitolBoth xylitol adapted animals and animals not previously exposed to xylitol were used.
Determination of Vitamin C Concentration by Titration.
Oxalic acid ingestion. It occurs naturally in many foods but excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base known as oxalate C 2 O 2 4 is a chelating agent for metal cations.
Typically oxalic acid occurs as the dihydrate with the formula C 2 H 2 O 4 2H 2 O. The absorption of 14C-labelled oxalic acid was studied in Wistar rats CD-1 mice and NMRI mice. Oxalic acid in solution was given to the animals by gavage either with water alone or with 0625 gkg body wt of xylitolBoth xylitol adapted animals and animals not previously exposed to xylitol were used.
Adaptation to xylitol diets enhanced the absorption and urinary excretion of the label. Oxalic acid is a dicarboxylic acid with the chemical formula C 2 H 2 O 4. Oxalic acid occurs in the cell sap of Oxalis and Rumex species of plants as the potassium and calcium salt.
In an aqueous solution oxalic acid is a weak acid that will only partially ionise. Oxalic acid has two acidic protons. The initial ionisation yields HC2O4- a weak.
OXALIC ACID 975383 1 8 Section. PRODUCT AND COMPANY IDENTIFICATION Product name. OXALIC ACID Other means of identification.
Cleaning product Restrictions on use. Reserved for industrial and professional use. Product dilution information.
Product is sold ready to use. Ecolab New Zealand 2 Daniel Place Te Rapa Hamilton New Zealand. Oxalic acid dihydrate C2H6O6 CID 61373 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Several hours prior to applying the oxalic solution we spray the bees with a 11 sugar solution to fill their honey stomachs and reduce ingestion of the upcoming oxalic treatment. Next we mix the oxalic acid in a 11 sugar water solution and evenly apply the solution to the bees.
It has been used for years in Europe. Oxalic acid is toxic because of its acidic and chelating properties. It may c ause burns nausea severe gastroenteritis and vomiting shock and convulsions.
It is especially toxic when ingested. As little as 5 to 15 grams 71 mgkg may be fatal to humans. Ulcerations of the mouth vomiting of blood and rapid appearance of shock convulsions twitching and cardiovascular collapse.
Page 3 of 8 MSDS - Oxalic acid Ingestion. Do NOT induce vomiting unless directed to do so by medical per sonnel. Never give anything by mouth to an unconscious person.
If large quantities o f this material are swallowed call a physician immediately. Loosen tight clothing such as a collar tie belt or waistband. Fire and Explosion Data Flammability of the.
Oxalic Acid Dihydrate Safety Data Sheet according to Federal Register Vol. 58 Monday March 26 2012 Rules and Regulations Date of issue. 11 01162018 EN English US Page 1 SECTION 1.
Identification Product form. Substance Substance name. Oxalic Acid Dihydrate CAS-No.
OXALIC ACID 75 - 95 144-62-7 not regulated as hazardous materials SECTION 4 - FIRST AID MEASURES IF IN EYES. Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do.
If eye irritation persists. Get medical advice attention. If medical advice is needed have container at hand.
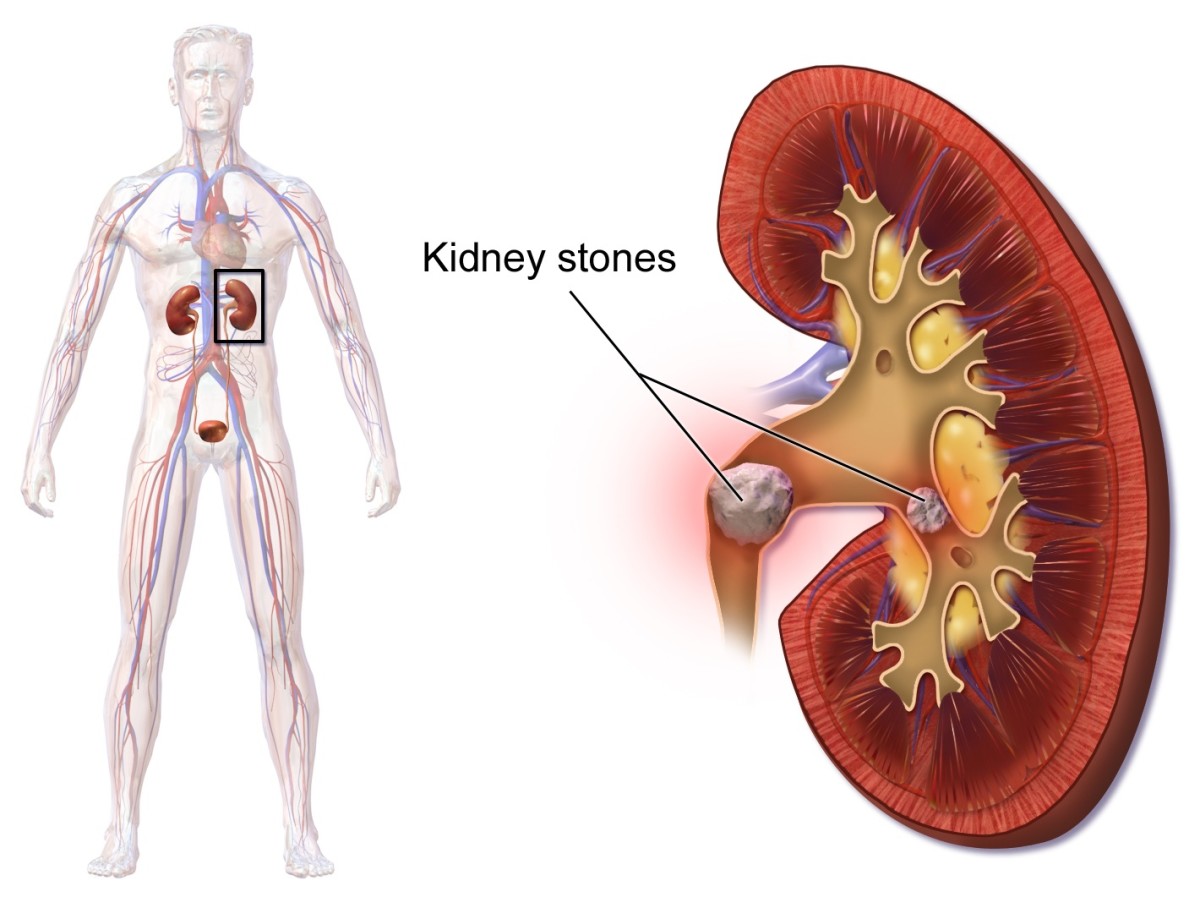
Drink milk or water and call a doctor. Consuming high amounts of oxalic acid can increase your risk of kidney stones making rooibos a good option for anyone with kidney problems. In addition to the metabolic acidosis and acute renal failure associated with glycolic acid and oxalic acid metabolites of ethylene glycol cause cerebral and meningo-encephalitis pulmonary edema and pneumonitis as well as myocardial hepatic and muscle pathology.
Because calcium oxalate is a very low solubility product significant hypocalcemia can result from precipitation of calcium by. Glutaric acid is the organic compound with the formula C 3 H 6 COOH 2. Although the related linear dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature the water-solubility of glutaric acid is over 50 ww.
Glutaric acid is naturally produced in the body during the metabolism of some amino acids including lysine and. Ulcerations of the mouth vomiting of blood and rapid appearance of shock convulsions twitching tetany and cardiovascular collapse may occur following ingestion of oxalic acid or its soluble salts. Systemic effects may be due to formation of calcium oxalate which is insoluble at physiological pH and can be deposited in the brain and kidney tubules.
Resultant hypocalcemia might. Ingestion of ethylene glycol may be an important contributor in patients with metabolic acidosis of unknown cause and subsequent renal failure. Expeditious diagnosis and treatment will limit.
Acid-base disorders are a group of conditions characterized by changes in the concentration of hydrogen ions H or bicarbonate HCO 3- which lead to changes in the arterial blood pHThese conditions can be categorized as acidoses or alkaloses and have a respiratory or metabolic origin depending on the cause of the imbalance. Diagnosis is made by arterial blood gas interpretation. Said to have 85 percent available chlorine.
Moderately toxic by ingestion. May irritate skin and eyes. Active ingredient in household dry bleaches.
Used in swimming pools as a disinfectant. See More Trichloroisocyanuric Acid Properties Seller Information. Send your message to this supplier.
Hiersun Pharmacy Co Limited. Iodate is toxic by ingestion. Determination of Vitamin C Concentration by Titration.
Equipment Needed burette and stand 100 mL volumetric flask 20 mL pipette 250 mL conical flasks 10 mL and 100 mL measuring cylinders Solutions Needed Potassium iodate solution. If possible dry 1 g of potassium iodate for several hours or overnight at 100C. Allow to cool and.
Glycolic acid and some oxalic and formic acids Salicylateaspirin. Sulfasalicylic acid SSA The first three of the nine causes of metabolic acidosis listed are medical or unusual physiological conditions. Strenuous exercise can cause temporary metabolic acidosis due to the production of lactic acid.
The last five causes result from the ingestion of specific substances. The active form of. Glycolic acid and some oxalic and formic acids Salicylateaspirin.
Sulfasalicylic acid SSA Acid metabolites from ingested chemical. The first three of the eight causes of metabolic acidosis listed are medical or unusual physiological conditions. Strenuous exercise can cause temporary metabolic acidosis due to the production of lactic acid.
The last five causes result from the ingestion. One exception is the ingestion of ethylene glycol. Given that the breakdown products of ethylene glycol include oxalate sufficient doses can produce calcium oxalate monophosphate crystal that deposits into tissues eg kidneys brain and precipitates in the urine.
Calcium oxalate monophosphate can be seen on urine microscopy and are shaped like dumbbells spindles ovals or. Rhubarb is a popular herb used in making desserts and is favored for its low sugar content and high fiber content. However only the barks of the plant are edible with its leaves having lethal toxins which include oxalic acid and anthraquinone glycosides.
When enough doses are ingested the toxins cause stomach aches nausea difficulty when. Oxalate or oxalic acid is a substance that can form insoluble salts with minerals including sodium potassium calcium iron and magnesium. These compounds are produced in small amounts in both plants and mammals.
All major groups of photosynthetic organisms produce oxalate. It is suggested that plants manufacture oxalate for a variety of functions including calcium regulation. Vitamin C also known as ascorbic acid exists as either ascorbic acid or ascorbate.
It is a water-soluble vitamin found in many fresh fruits and vegetables and is essential for a variety of physiologic functions including formation of collagen and catecholamines and carnitine and peptide synthesis 1. If swallowed get immediate medical attention. Do not induce vomiting.
Never give anything by mouth to an unconscious person. Ethylene glycol is oxidized to oxalic acid which results in the deposition of calcium oxalate crystals mainly in the brain and kidneys. Early signs and symptoms of EG poisoning may resemble those of alcohol intoxication.
Later the victim may experience.