
Nitrogen dioxide is produced for the manufacture of nitric acid. When inhaled or ingested these metals can cause.
Nitrogen dioxide is a.
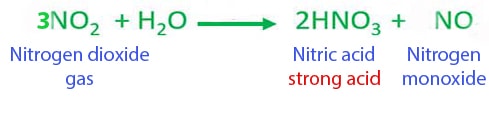
Nitrogen monoxide to water. Nitrogen compounds have a very long history ammonium chloride having been known to HerodotusThey were well known by the Middle Ages. Alchemists knew nitric acid as aqua fortis strong water as well as other nitrogen compounds such as ammonium salts and nitrate salts. The mixture of nitric and hydrochloric acids was known as aqua regia royal water celebrated for its ability to dissolve.
Nitrogen dioxide is a chemical compound with the formula NO 2. It is one of several nitrogen oxides. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizers.
At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity. Nitrogen dioxide is a.
We simulated the waterCu100 interface using 48 explicit. Formation of carbonnitrogen bonds in carbon monoxide electrolysis. 11 846851.
Cement plants are a significant source of sulfur dioxide nitrogen oxide and carbon monoxide which are associated with the following health and environmental impacts. Nitrogen oxide NO x can cause or contribute to a variety of health problems and adverse environmental impacts such as ground-level ozone acid rain global warming water quality deterioration and visual impairment. Oxides of nitrogen are a mixture of gases that are composed of nitrogen and oxygen.
Two of the most toxicologically significant compounds are nitric oxide NO and nitrogen dioxide NO 2Other gases belonging to this group are nitrogen monoxide or nitrous oxide N 2 O and nitrogen pentoxide NO 5. Nitrogen dioxide is produced for the manufacture of nitric acid. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen.
On a small scale pure nitrogen is made by heating barium azide BaN 3 2. Carbon Monoxide CO Methanol CH 3 OH Moisture H 2 Ov Nitric Oxide NO Nitrogen Dioxide NO 2 Oxygen O 2 Sulfur Dioxide SO 2 Total Sulfur Total Volatile Hydrocarbons Back to Top Gas Chromat ographs Model Description Detectors Falcon GC Ultra-Fast Gas Chromatograph for Process and Lab Applications A spiration to inlet via sample pump Process online via sample valve for gas or. Other natural sources of carbon monoxide are marsh gases which are also called methane and produced by plants decomposing under water marine algae kelp and seed germination growth.
Carbon monoxide is a major component of motor vehicle exhaust fumes and is emitted into the atmosphere by cars trucks boats and aeroplanes. Nitrogen is a non-flammable air gas that forms 78 of the earths atmosphere. Valued for its inert properties in its gaseous form nitrogen displaces air and therefore reduces or eliminates the oxidation of materials.
It is also used as an assist gas for laser cutting. Given the extremely low temperatures of its liquid state nitrogen is an. Refrigerated cryogenic nitrogen is a colorless odorless liquid.
Gaseous nitrogen is used in food processing in purging air conditioning and refrigeration systems and in pressurizing aircraft tires. Liquid nitrogen is used to freeze foods to preserve whole blood and other biologicals and as a coolant. The term nitrogen oxides NOx describes a mixture of nitric oxide NO and nitrogen dioxide NO 2 which are gases produced from natural sources motor vehicles and other fuel burning processes.
Nitric oxide is colourless and is oxidised in the atmosphere to form nitrogen dioxide. Nitrogen dioxide has an odour and is an acidic and highly. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms.
It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Nitric oxide NO also called nitrogen monoxide. The gas is almost insoluble in water but it dissolves rapidly in a slightly alkaline solution of sodium sulfite forming the compound sodium dinitrososulfite Na 2 NO 2 SO 3.
It reacts rapidly with oxygen to form nitrogen dioxide NO 2. Nitric oxide is a relatively unstable diatomic molecule that possesses a free radical ie an. The Clean Air Act requires EPA to set national ambient air quality standards NAAQS for carbon monoxide and five other pollutants considered harmful to public health and the environment the other pollutants are ozone particulate matter nitrogen oxides sulfur dioxide and lead.
The law also requires EPA to periodically review the standards to ensure that they provide adequate health and. Invisible pollutants nitrogen oxides sulfur dioxide volatile organic compounds carbon monoxide also pose a threat to health. Carbon monoxide displaces oxygen in red blood cells leading quickly to death if the carbon monoxide concentration is high.
Nitrogen oxides sulfur dioxide and volatile organic compounds irritate the sensitive tissues of the airway. They are particularly irritating. These six pollutants are carbon monoxide lead nitrogen oxides ground-level ozone particle pollution often referred to as particulate matter and sulfur oxides.
Carbon Monoxide ATSDR Toxic Substances Portal. Carbon Monoxide EPA Website external icon. Interaction Profile Carbon Monoxide Formaldehyde Methylene Chloride Nitrogen Dioxide Tetrachloroethylene.
That includes carbon dioxide carbon monoxide and nitrogenthree greenhouse gases that are unfortunately responsible for climate change. During the explosion these metal salts do not burn up They are still metal atoms and many of them are end up as aerosols that poison the air the water and the soil writes GrrlScientist. When inhaled or ingested these metals can cause.
The widespread use of nitrogen in industrial processes makes it vital for operators to understand and employ the most efficient nitrogen generation techniques. While nitrogen occurs freely in nature it is not readily available in its most useful form. Nitrogen is typically mixed with other component gases of air that may have an undesirable effect on industrial manufacturing processes.
Nitrogen gas purity refers to the proportion of a nitrogen gas that is present in a sample taken from its stream when compared to impurities present. Nitrogen gas can be categorized as high purity or low purity depending on the ratio of pure gas to the contaminants like oxygen water vapor carbon monoxide and carbon dioxide. Chemical Physical and Thermal Properties of Carbon Monoxide - CO Related Topics.
Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Acetone - Thermophysical Properties - Chemical physical and thermal properties of acetone also called 2-propanone dimethyl ketone and pyroacetic acid. The six common air pollutants covered by the Clean Air Act ground-level ozone particulate matter carbon monoxide lead sulfur dioxide SO2 and.
Propane Oxygen Carbon Dioxide Carbon Monoxide Water Heat. 2 C 3 H 8 9 O 2 4 CO 2 2 CO 8 H 2 O heat. So propane does cause carbon monoxide generation when there is incomplete combustion.
Does Burning LPG Produce Carbon Monoxide CO Burning LPG can produce carbon monoxide if there is incomplete combustion. All gas appliances domestic and industrial. The data on components of engine exhausts such as carbon monoxide nitrogen oxides and sulfur dioxide can be used to indicate the presence of engine exhaust but cannot be used to apportion exposures.
Thus information on single components reported in these studies cannot be used to rank relative exposures to total engine exhaust reliably due to the variable relationships among the. Nitrogen oxides NOx. These pollutants form ground level ozone and particulate matter secondary.
Also harmful as a primary pollutant NOx can cause lung irritation and weaken the bodys defenses against respiratory infections such as pneumonia and influenza. This odorless colorless and poisonous gas is formed by the combustion of fossil fuels such as gasoline.