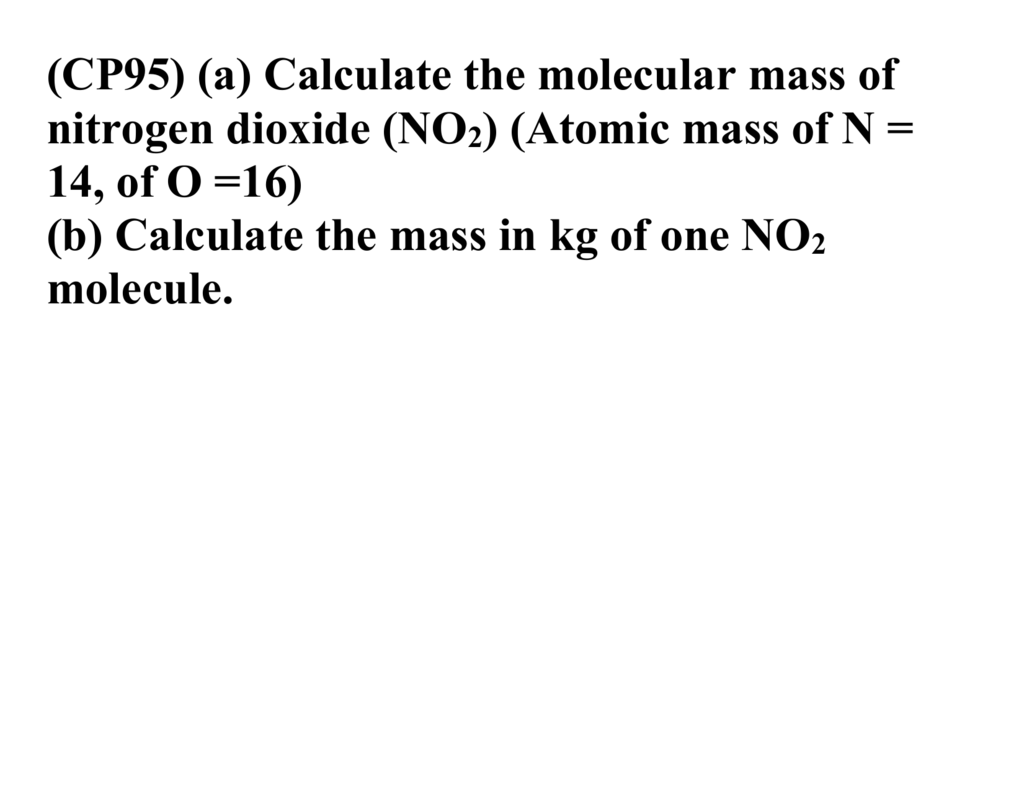
Ammonia NH 3 17031 u. Both react with water to form nitric acid.
Nitrogen Trifluoride NH3 Ammonia NH4Br Ammonium Bromide NH4Cl Ammonium Chloride NH4NO2 Ammonium Nitrite NH4NO3 Ammonium Nitrate NH4OH Ammonium Hydroxide NiNO32 NickelII Nitrate NI3 Nitrogen Triiodide NiBr2 Nickel Bromide NiCl2 NickelII Chloride NiO NickelII Oxide NiSO4 Nickel Sulfate NO2 Nitrogen Dioxide NO3 Nitrate Ion OF2 Oxygen.
Nitrogen dioxide mass. Nitrogen dioxide is a chemical compound with the formula NO 2. It is one of several nitrogen oxides. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizers.
At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity. Molecular weightmolar mass of NO 2.
Density of Nitrogen dioxide. Boiling Point of Nitrogen dioxide. Melting Point of Nitrogen dioxide.
Nitrogen dioxide Sources NO 2. Over 98 percent of man made N0 emissions result from Combustion with the majority due to stationary sources. Combustion generated oxides of nitrogen are.
The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon and hydrogen. Nitrogen dioxide is an acrid corrosive brown gas. Both compounds may be easily prepared by decomposing a dry metal nitrate.
Both react with water to form nitric acid. Dinitrogen tetroxide is very useful for the preparation of anhydrous metal nitrates and. They found just a 3 per cent reduction in nitrogen dioxide NO2 levels over this time and insignificant drops in levels of ozone O3 which can damage the lungs and tiny particles of dirt.
The strong correlation between the unexplained sulfate mass and aerosol liquid water content during these events. Then add the unit as gramsmole you will get the molecular mass of the substance. Molecular mass of Nitrogen.
Nitrogen is a chemical element that has the symbol N and atomic number 7 and atomic mass 1400674µ. For nitrogen the mass of the N 2 molecule is. N 2 1401 1401.
N 2 2802. Thus the molecular mass of a nitrogen molecule 28 u. Nitrogen is a chemical element with atomic number seven and atomic weight 14.
Its symbol is N. With these facts about nitrogen let us learn about its chemistry physical properties atomic mass and much more. 26 Facts about Nitrogen.
It has a melting point of 20986 C 3458 F and a boiling point of 1958 C 3204 F. Nitrogen dioxide NO 2 and formaldehyde HCHO profiles retrieved from remote sensing observations are used to evaluate the global chemistry transport model CHASEROverall CHASER has demonstrated good skills in reproducing the seasonal climatology of NO 2 and HCHO on a local scale at sites in South and East Asia. Around mountainous terrains the model performs better on a regional.
Variations in the isotope-amount ratio n15 Nn14 N are used to determine sources of nitrogen contamination in the atmosphere oceans groundwater and rivers where the isotopic composition of a contaminant molecule preserves evidence of the nitrogen sources and processes involved in its creation. An example is nitrate derived from artificial fertilizer manure power-plant emissions or. Carbon provides both an energy source and and the basic building block making up about 50 percent of the mass of microbial cells.
Nitrogen is a crucial component of the proteins nucleic acids amino acids enzymes and co-enzymes necessary for cell growth and function. To provide optimal amounts of these two crucial elements you can use the carbon-to-nitrogen CN ratio for each of your. Relative atomic mass A r.
1400643 1400728 see notes g r. NO and then the dioxide NO 2. When heated under pressure with hydrogen in the presence of a suitable catalyst ammonia forms Haber process.
Nitrogen is fixed from the atmosphere by bacteria in the roots of certain plants such as clover. Hence the usefulness of clover in crop rotation. Image adapted with.
The name nitrogen was therefore proposed from the observation again first made by Cavendish that if the gases sparked with oxygen and then the resulting nitrogen dioxide gases passed through alkali nitre otherwise known as saltpetre or potassium nitrate is formed. The word nitrogen therefore means nitre former. The derivatives of the word azote still survive today.
The compound used to. With every episode of gas and especially burping cattle release methane which is 23 times more harmful than carbon dioxide the main greenhouse gas in car emissions. Besides cows gas their manure can be problematic.
The phosphorus and nitrogen in cow manure after its applied to farmland as fertilizer can run off with rainfall into local waterways including Lake Erie contributing. Carbon hydrogen oxygen nitrogen and phosphorus as elements and compounds makeup 97 of the mass of our bodies and are more than 95 of the mass of all living organisms. In addition to these about 15 to 25 other elements are needed in some form for the survival and good health of plants and animals.
These elements or mineral nutrients are always in circulation moving from non-living to. As the organic mass in landfills decompose methane gas is released. Along with methane landfills also produce carbon dioxide and water vapor and trace amounts of oxygen nitrogen hydrogen and non methane organic compounds.
These gases can also contribute to climate change and create smog if left uncontrolled. The creation of landfills typically means destroying natural habitats. Based on the relative volumes of the gases in Earths atmosphere nitrogen is actually more than 3 times more than oxygen.
Because the troposphere is the lowest atmosphere layer it contains 75 percent of the atmospheres mass. From largest to smallest Earths atmosphere composition contains nitrogen oxygen argon CO 2 and trace gases. For more information on NASAs long-term measurements of nitrogen dioxide please see this page.
During the quarantine roads and transportation hubs are emptier. Street traffic cleared out near the Wuhan train station during the quarantine. It is no surprise that road traffic in Chinas major cities has been lighter as many people have been forced to.
Nitrogen N 2 is at standard conditions a colorless odorless gas. The gas makes up the major portion of the atmosphere but will not support life by itself. Refrigerated cryogenic nitrogen is a colorless odorless liquid.
Gaseous nitrogen is used in food processing in purging air conditioning and refrigeration systems and in pressurizing aircraft tires. Liquid nitrogen is used to freeze. Nitrogen is an inert gas with many industrial applications.
It is liquefied by cooling at -3208 F -196 C7715 K. It is mainly found in the atmosphere where it accounts for 78 by volume of the air we breath. But nitrogen is also found in the Earths crust to a limited extent.
In the form of nitrates etc in organic form in the living or dead plants and organisms in mineral. Air is a mixture of several gases where the two most dominant components in dry air are 21 vol oxygen and 78 vol nitrogen. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol.
Since both of these elements are diatomic in air - O 2 and N 2 the molar mass of oxygen gas is 32 gmol and the molar mass of nitrogen gas is 28 gmol. The average molar mass is. 92 bars Surface density.
Kgm 3 Scale height. 159 km Total mass of atmosphere. 48 x 10 20 kg Average temperature.
737 K 464 C Diurnal temperature range. 03 to 10 ms surface Mean molecular weight. 4345 Atmospheric composition near surface by volume.
965 Carbon Dioxide CO 2 35 Nitrogen N 2 Minor ppm. Feng and collaborators at Oregon State the University of Central Florida and the University of Pittsburgh found that putting fluorine atoms into palladium-nitrogen-carbon catalysts had a number of positive effectsincluding keeping the power-dense cells stable for nearly 6000 hours. A catalyst is a substance that increases the rate of a reaction without itself undergoing any permanent.
The box is empty so inside it would be about 78 per cent nitrogen and 21 per cent oxygen with trace amounts of carbon dioxide. While few people seem to know that carbon dioxide makes up only 004 per cent of the Earths atmosphere Im often told that carbon dioxide is a greenhouse gas. They do shout the bit about it being a fact as though that makes it special because it.
Nitrogen Trifluoride NH3 Ammonia NH4Br Ammonium Bromide NH4Cl Ammonium Chloride NH4NO2 Ammonium Nitrite NH4NO3 Ammonium Nitrate NH4OH Ammonium Hydroxide NiNO32 NickelII Nitrate NI3 Nitrogen Triiodide NiBr2 Nickel Bromide NiCl2 NickelII Chloride NiO NickelII Oxide NiSO4 Nickel Sulfate NO2 Nitrogen Dioxide NO3 Nitrate Ion OF2 Oxygen. Nitrogen Dioxide NO 2 46006 u. 1 g mol 1 46006 g mol 1.
Ammonia NH 3 17031 u. 1 g mol 1 17031 g mol 1. Glucose C 6 H 12 O 6 180156 u.
1 g mol 1 180156 g mol 1. Atomic Weight to Molar Mass. The atomic weight aka relative atomic mass A r is a dimensionless quantity having the numeric value of the average atomic mass.