
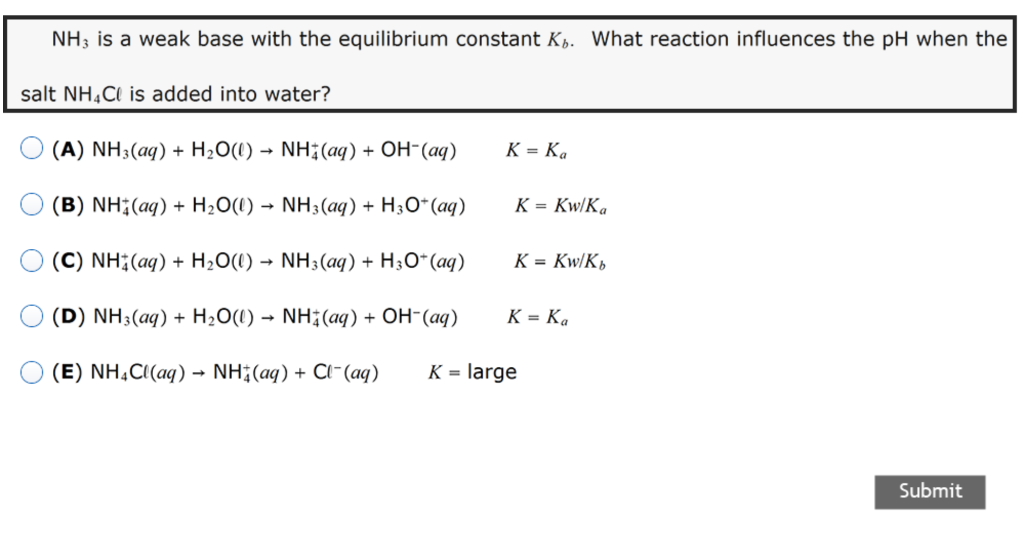
What is the pH of a 043M solution of NH4Cl. We use the molar ratio of reactant in a balanced chemical reaction to understand how much product will be created under ideal conditions.
What volume of 01 moldm-3 HCL acid would be required to dissolve 23 grams of calcium carbonate equation CaCO32HCL—–CaCl2CO2H20 WATER chemistry 101.
Nh4cl reaction with water. Answer 1 of 7. We will get this as the equation NH4Cl H2O —- NH4OH HCl Basically We have NH4 and Cl- as ions And OH- and H and ions from water Thus we get NH4OH and HCl. Reaction 2 NH4Cl s NH41 aq Cl-1aq Reaction 3 NH3 aq HCl aq NH4Cl aq.
Measure 500 mL of distilled water using a graduated cylinder. Add this to the. Calorimeter and place the cover on it.
Place the temperature probe through the lid of the. Calorimeter and into the water. Press ENTER on the calculator.
Wait for about 3-4 points to appear on the calculator. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above.
Use uppercase for the first character in the element and lowercase for the second character. Fe Au Co Br C O N F. Ionic charges are not yet supported and will be ignored.
Home Reactions Blog. Check the balance Aluminum chloride react with ammonia and water to produce aluminium hydroxide and ammonium chloride. Thermodynamic properties of substances The solubility of the substances Periodic table of elements.
AlCl3 3 NH3 3. Type of Chemical Reaction. For this reaction we have a chemical reaction.
In this reaction Ammonium chloride and Sodium hydroxide are reacting to form Ammonia Water and Sodium chloride. Be sure to count all of the hydrogen atoms H on each side of the equation. For a complete explanation watch.
As you see in the above reaction NH 4 donates the proton to the water molecule and forms the H 3 O or H ion. The presence of an H ion makes the solution slightly acidic. Heres what Arrhenius said for acid-Arrhenius theory for acid.
According to this a compound is an acid when it gives H ions on dissolving in an aqueous solution or increases the amount of hydrogen ion in the. Answer 1 of 5. In order to understand this properly let us do a practical example.
What is the pH of a 043M solution of NH4Cl. Kb of NH3 1810-5 Do some preliminary calculations. We eventually want H3O to calculate pH so determine the Ka of NH3 from Kb The Kb for NH3 is 1810.
Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases.
It is also found around some types of volcanic vents. Which one of the following compounds will NOT be soluble in water. K2S MgCl2 BaSO4 LiOH NaNO3.
Based on the solubility rules will the following reaction occur because a precipitate will form. 2 AgNO3aq NiC2H3O22aq – 2 AgC2H3O2 NiNO32 no both need more info yes NiNO32 yes AgC2H3O2 yes both. According to the activity series for metals will the following reaction.
Double-decomposition processes all involve the reaction of sodium chloride the cheapest chlorine source with an. Transferred to acid resistant crystallizing pans concentrated and cooled to effect crystallization. The crystalline NH4Cl is washed with water to remove sulfate and dried to yield a product of high purity.
Weston CW et al. Kirk-Othmer Encyclopedia of. The electrolytic decomposition of water gives H 2 and O 2 in the ratio of a 1.
2 by volume b 2. 1 by volume c 8. 1 by mass d 1.
In the decomposition of lead II nitrate to give lead II oxide nitrogen dioxide and oxygen gas the coefficient of nitrogen dioxide in the balanced equation is a 1 b 2 c 3 d 4. Fatty foods become rancid due to the process of. The reaction between the two substance shoes that all substances are aqueous and can dissolve in water Identify the solid product formed if any from the reaction of K3PO4 and CuCl2.
Here a strong base reacts with a weak acid to form salt and water. But since the reaction uses a strong acid the pH at the endpoint will be towards acidic ie below 7. Here the salt formed NH4Cl is slightly acidic.
So indicators changing color at lower pHs are employed. During the reaction a known concentration of strong acid. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Fe Au Co Br C O N F.
Ionic charges are not yet supported and will be ignored. NH4Cl aq Youd rewrite it like this changes have been highlighted. Add 574 mL of a 1M solution of propylmagnesium bromide in THF to a sealed 500 mL fllask under an atmosphere of nitrogen cooled to 5 C in an ice-water bath.
Add 611 mL 2-pentanone over the course of 30 min via syringe while maintaining internal temperature below 25 C. Once all the 2-pentanone is added stir for four. We use the molar ratio of reactant in a balanced chemical reaction to understand how much product will be created under ideal conditions.
You may also be asked to compare your yield calculations with actual yield which captures the impact of impurities process inefficiencies. Weigh how much of each reactant you are going to put into the process look up molar weight and mole ratio number. Sodium fluoride in drinking water and toothpaste comes from the reaction of sodium and.
CuSO4 x 5H2O arrow CuSO4 C. CuSO4 arrow CuSO4 x. If 265 g of CuNO3 is dissolved in water to make a 0480 M solution what is the volume of the solution in milliliters Ammonium chloride NH4Cl decomposes when it is heated.
A How many components and phases are present when the salt is heated in an otherwise empty c. This reaction is still sometimes referred to by its old name hydrolysis water splitting which is literally correct but tends to obscure its identity as just another acid-base reaction. Reactions of this type take place only to a small extent.
A 01M solution of. The sodium hypochlorite reaction with water is similar to gaseous chlorine forming sodium hydroxide NaOH hypochlorous acid HOCl hypochlorite ion OCl- and hydrogen ion H. This is usually expressed as.
2NaOCl 2H2O – 2NaOH HOCl OCl- H The hydrochloric acid ionizes the same as gaseous chlorine and is pH dependent. HOCl – H OCl-The main difference between chlorine gas. NH4Cl is an inorganic compound with the chemical name Ammonium Chloride.
It is also known as sal ammoniac the salt of ammonia and hydrogen chloride. It is a by-product of sodium carbonate. It has diuretic and expectorant effects.
In its pure form it is crystalline salt white. This compound is highly water-soluble and mildly acidic. It is used in veterinary medicine in the prevention of.
When 205 moles of CH4 are mixed with 503 moles of O2 in the reaction below the limiting reactant is CH4 2O2 - CO2 2H2O. CH42O2—–CO22h2O Use this balanced equation to calculate the mass of water formed when 32g of methane burns. Write a balanced equation for the dissociation of each of the following strong electrolytes in water.
Indicate whether aqueous solutions of each of the following solutes contain only ions only molecules molecules and a few ions. NH4Cl a strong electrolyte O. IronII nitric ironII water carbon carbonate acid nitrate dioxide 39.
Overall balanced equation for reaction of NH 4 2 S with HBr. NH 4 2 S 2 HBr H 2 S 2 NH 4 Br Ammonium sulfide Hydrogen bromide Hydrogen sulfide Ammonium bromide Oxidation Numbers 41. For questions on oxidation number read the symbol x as the oxidation number.
What volume of 01 moldm-3 HCL acid would be required to dissolve 23 grams of calcium carbonate equation CaCO32HCL—–CaCl2CO2H20 WATER chemistry 101.