Calculate the concentration of NaOH in the following way. Choisissez bien votre espace de travail pour la fabrication de vos savons.
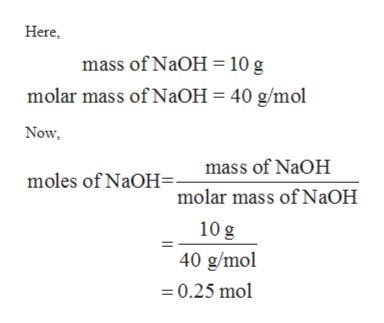
PH of buffer NaOH.
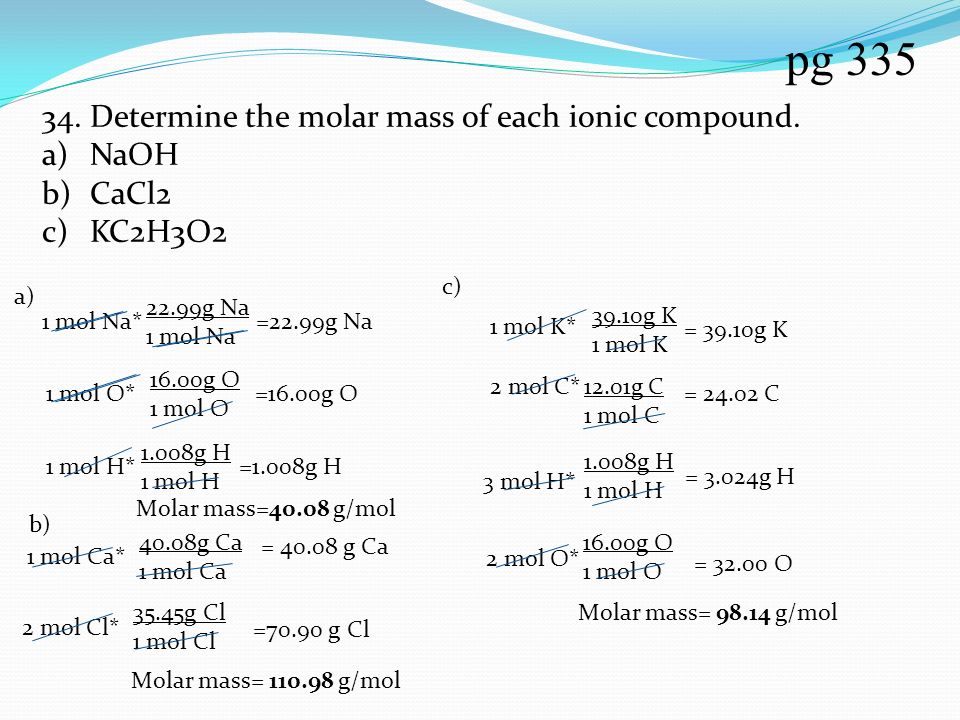
Mw of naoh. MW of NaOH 10 gmole Prepare 008 M Molarily of NaOH in 1 L distilled water. How many grams of NaOH are needed 6 Q3. 1221 16 Prepare 1200 ppm of NaOH in 1 L distilled water.
How many grams of NaOH are needed Q2. MW of MgOH2 58 gmole Prepare 005 N Normality of MgOH2 in 1 L distilled water. How many grams of MgOH2.
This problem has been. Buffer Calculator Dear researchers we know you must have lots of work to do for your research. Considering about it there is a sweet guy in my company developing this buffer calculator online so that you have no worries on buffer calculating.
It can save your time on. Weigh 08 g of dried KHP MW 20423 gmol into an Erlenmeyer flask and dissolve in 50-75 mL of distilled water. Record the amount of KHP and water used.
Add 4 drops of indicator into the flask and titrate to the first permanent appearance of pink. How many moles of KHP react with NaOH. In this reaction as well one mole of KHP completely reacts with one mole of NaOH.
C 4 H 6 0 2 MW 11809 Add 02M NaOH to desired pH. Dilute with ddH 2 0 to desired molarity. Maleate Buffer sodium hydrogen maleate buffer pH 52-68.
02M 1 liter Maleic acid MW 12114 232 gm ddH 2 0 to make 1 liter. Adjust pH with 01M Na0H. Dilute with ddH 2 0 to desired molarity.
Imidazole Buffer pH 62-78. Imidazole 02M 13621 C 3 H 4 N 2 MW 68. NaOH KHC8H4O4 NaKC8H4O4 H2O Chemical Equation Balancer.
How do you standardize NaOH with KHP. Weigh 08 g of dried KHP MW 20423 gmol into an Erlenmeyer flask and dissolve in 50-75 mL of distilled water. Record the amount of KHP and water used.
Add 4 drops of indicator into the flask and titrate to the first. MW 2042 gmol required to give a 25 mL titration using 010 M NaOH. Pre-calculations are done to help plan the experiment even when experimental design is not your job per se they help you understand how the experiment works and why the specified amounts are used.
They are not part of calculations that are used to determine the. Weigh 08 g of dried KHP MW 20423 gmol into an Erlenmeyer flask and dissolve in 50-75 mL of distilled water. Record the amount of KHP and water used.
Add 4 drops of indicator into the flask and titrate to the first permanent appearance of pink. Near the endpoint add the NaOH dropwise to determine the total volume most accurately. Calculate the concentration of NaOH in the following way.
NaOH NaOH ts cc N where 2 1 1 N i NaOH NaOH i cc s N. Here N is the number of measurements N 3 and t is the so-called Students coefficient assuming that the confidence level is 95 and that the number of. NaOH KOH LiOH MW.
WB490000 NaOH and basic salts 5611 KOH 1310-58-3 TT2100000 KOH 2395 LiOH 1310-65-2 OJ6307070 LiOH METHOD. 7401 Issue 2 EVALUATION. 15 February 1984 Issue 2.
15 August 1994 OSHA. 2 mgm3 NaOH NIOSH. C 2 mgm315 min NaOH.
Group I Pesticide ACGIH. C 2 mgm3 NaOH PROPERTIES. Moles of NaOH mass NaOHMW of NaOH 750 g NaOH39997 gmol 1875 mol NaOH.
C moles of NaOHV. V moles of NaOHC 1875 mol NaOH0500 mol NaOHL 3750 L of 0500 M NaOH. V 3750 liters of 0500 M NaOH.
Upvote 0 Downvote Add comment More. 50 66 Experienced Physics Teacher for Physics Tutoring. MM molar mass MW or FW The equation can also be used to find n the number of moles of solute if M the molarity and V the volume in litres are known and V if n and M are known.
N MV V n M-4-a Calculate the molarity of a solution of 025 mole of NaOH in 50 L of solution. Molarity 0050 025 mole M 50 L b Calculate the molarity of a solution of 48 mole of. Answer 1 of 15.
1N HCL - add 3646 gm of HCL in 1 litre of water Molar mass of HCL 3646 gmole 3646 g of HCL is equivalent to 3067 ml of HCL Volume MassDensity 3646119 3067 Density of HCL is 119 gml Percent Concentration of Most Conc HCL is 375 3067. Equilibrium Expression Value of K or K. Vas bo Not tlo1h 2 Hoon 7200X10 TABLE 77 Net Ionic Equation Solution for Hydrolysis Na CO Na CHO NGHO Notensi NHCI N.
ZnCI ZnOH2 20 KAKSO. A13 340 All 35 35 163 0215 no BUFFERS Mass of NaCHO 3 HO. MW 163 gmol Mols of NaCHO 3 H0 pH of buffer solution.
PH of buffer HCI. PH of buffer NaOH. 10 NaOH means 10g of NaOH in 100 ml.
Normalitymolalitynumber of protons used molalitymassRAM x volumeL 10g40x01 25M therefore normality25Mx1 25N Thanks. Not Helpful 27 Helpful 50. When 356 g hcl is present in 500ml of solution what will be its normality.
It should be as follows. H 1008 Cl 3545 1 M 3645 V1 ew. Filter extraction solution 2 NaOH-3 Na 2CO 3.
Dissolve 20 g NaOH and 30 g Na 2CO 3 in deionized water to make 1 L of solution. See SPECIAL PRECAUTIONS. Polyvinyl chloride PVC filter 50-µm pore size 37-mm diameter in polystyrene cassette filter holder FWSB MSA or VM-1 Gelman or.
Reagents MW gmol MP ºC BP ºC Density gmL Molarity molL cyclohexanol 10016 23-25 161 0965 NA sulfuric acid 9807 NA NA NA 9 M water 18 0 100 100 NA Products MW gmol MP ºC BP ºC Density gmL Molarity molL cyclohexene 8215 -104 83 0811 NA FOR YOUR SAFETY 1. Wear gloves at all times when handling the aqueous sulfuric acid at the beginning of the reaction and. Making 500ml of 1M Tris pH 82 would go like this Tris MW 12114.
1M 12114g1000ml 1M 6057g500ml Dissolve 6057g of Tris in 450ml H 2O in a 500ml beaker. Titrate the solution to 82 using either HCl acid or NaOH base as needed. Never counter the effects of spilled acid with strong lye such as KOH or NaOH.
Use water or a weak base such as diluted sodium hydrogen carbonate NaHCO3 instead. Helpful 19 Not Helpful 7. Do not dissolve materials for fun or whatever other reason unless you know exactly what you are doing.
In this way you may create extremely dangerous products such as poisonous or explosive. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol. C 4 H 10 O MW 74 is 118 C 244 F.
In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. The boiling point of pentane C 5 H 12 MW 72 is 36 C 97 F close to the. 溶解度 水に溶解0 042gmL 100 347g mL 吸湿性潮解性があるので秤量はすばやく行う.
If low MW PVA is used prepare an 8 solution by placing 920 cm 3 of water into a tall 1 dm 3 beaker. Measure out 80 g of low MW PVA and add this slowly to the beaker of water with stirring. In each case heat the mixture gently stirring occasionally until the solution clears.
Avoid boiling the solution. After cooling this solution can be poured into suitable smaller containers which can. Buffers used were as follows.
5 TAPS-PEG 8000 50 mM TAPS-NaOH at pH 85 RT 25 mM MgCl 2 40 PEG 8000 or 5 TAPS-DMF 50 mM TAPS-NaOH at pH 85 RT 25 mM MgCl 2 50 DMF. For library production the reactions were incubated for 7 min at 55C and then 5 μL 02 SDS final 002 was added and Tn5 was inactivated for 7 min at room temperature or 55C. Una solución de 2 M de NaOH tiene 1 molécula de hidróxido por lo que 2 M x 1 2 N.
Método 2 de 2. Obtener la normalidad a partir del peso equivalente 1. Encuentra la masa molar total del compuesto.
Observa la fórmula química del compuesto y ubica cada uno de los elementos en la tabla periódica. Anota las respectivas masas molares y multiplícalas por la cantidad. 120 g de soude pure solide hydroxyde de sodium NaOH pour la réaction chimique qui se trouve en magasin de bricolage.
Les huiles essentielles que vous préférez facultatives comme des huiles au poivre au citron à la rose à la lavande des colorants des parfums des décorations etc. Choisissez bien votre espace de travail pour la fabrication de vos savons. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3.
MW 92 bp 111 C 2318 F. The ability of phenols to form strong hydrogen bonds also enhances their solubility in water. Phenol dissolves to give a 93 percent solution.