If you have one molecule of water H 2 O you can see that two H atoms react with one O atom to form this compound. Here we will prepare 100 ml of 10M NaOH solution.
The long chain fatty acids found in fats have low saponification value because they have a relatively fewer number of carboxylic functional groups per unit mass of the fat and therefore high molecular weight.
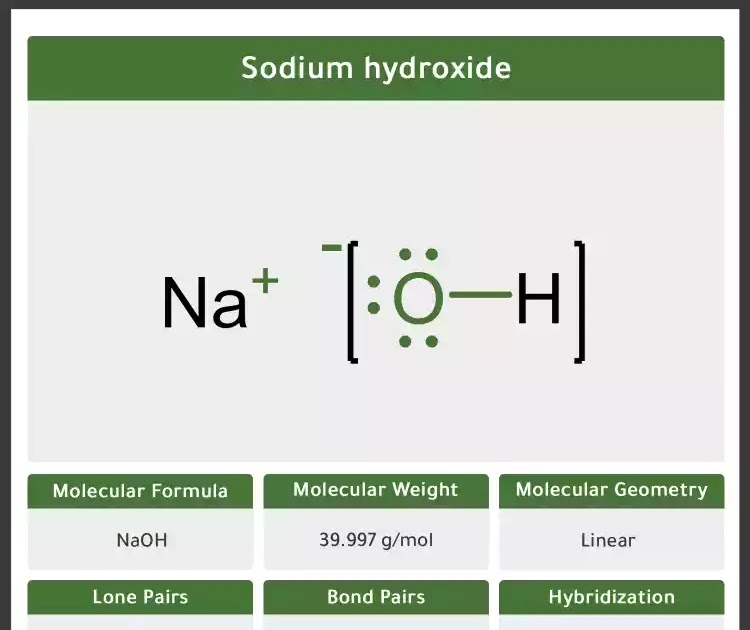
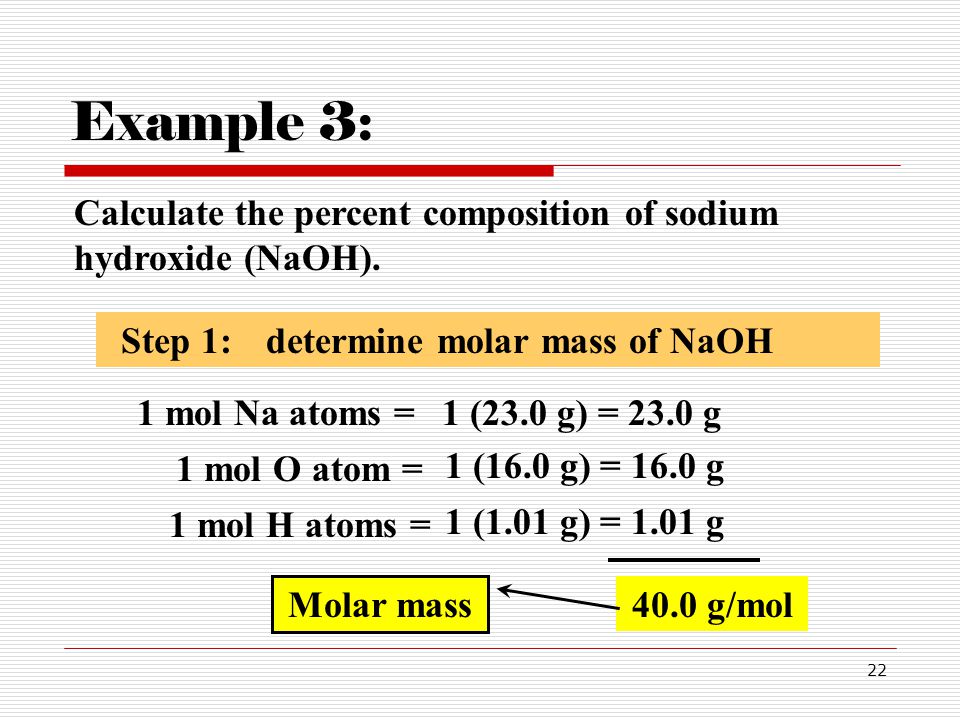
Molecular weight naoh. Molar mass of NaOH 3999711 gmol This compound is also known as Sodium Hydroxide. Convert grams NaOH to moles or moles NaOH to grams. 2298977 159994 100794.
Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. The molecular weight of sodium hydroxide is 40 gmol. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps.
To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. Visit BYJUS for more information. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide.
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles NaOH or 3999711 grams. Note that rounding errors may occur so always check the results.
Use this page to learn how to convert between moles NaOH and gram. Type in your own numbers in the form to convert the units. The molecular weight of NaOH is 40.
That means you need to dissolve 40 g of NaOH in water to obtain a 1 liter of 1M or 1N NaOH solution. To prepare a 10M NaOH solution you need to dissolve 10 times more NaOH ie 400 g of NaOH for 1 L solution. Here we will prepare 100 ml of 10M NaOH solution.
Therefore we need to take 40 g of NaOH. Dissolution of NaOH is a hyperthermic reaction. Molecular Weight of Hydroxide.
Monoisotopic mass of Hydroxide. Structure of Hydroxide OH Structure of Hydroxide. Uses of Hydroxide OH Hydroxide is used to produce fuel cells.
Used to produce disinfectants. Used as food preservatives to prevent bacteria and mold growing in food. Used in the paper.
NaOH 50 194 Molar 250 ml of 50 NaOH weighs 3793 gm. Density 152 gmml. Strength 50-52 Density 152 Molecular Weight 400 1 liter 1520 gm.
Examples of molecular weight computations. Definitions of molecular mass molecular weight molar mass and molar weight. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
1 u is equal to 112 the mass of one atom of carbon-12. The mass of one mole of a substance is equal to that substances molecular weight. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams.
The amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon-12. The chemical changes observed in any reaction. 3 MultiplyH 1.
0 mol dm-3 sulphuric acid H2SO4 reacted with 25 cm3 of NaOH. 150g H2SO4 Molecular Weight Search Help Search options step 1 Back to search You may search for species based on molecular weight values in two ways. Specify a single value.
The system will search for values within 0. 02 amu 1 mol AgCl 1 mol Cl mol Cl in 1. For 12 C the atomic mass is exactly 12u since the.
Considering that the molecular weight of KHP is MWKHP 20423 gmol the concentration of the KHP solution is. 48149 g 0250 L 00943035 M 943035 mM KHP 20423 gmol c The titration standardization results using 2500 mL aliquots of the KHP solution are summarized in Table 1 below. Trial 1 Trial 2 Trial 3 Initial volume mL 150 050 2460 Final volume.
Molecular weight of NaOH 40 Then During the preparation of molar solution the weight to be taken is calculated according to the following method using the formula -. As you move from left to right and downward on the periodic table the mass of one mole of a given element or its molecular weight MW is given in the corresponding box for that element usually at center bottom. An example helps make sense of this definition.
If you have one molecule of water H 2 O you can see that two H atoms react with one O atom to form this compound. But because the. Assuming your 20 is weight on weight just add 80 grams of water 80 mL at 20 degrees Celsius to 20 grams of NaOH.
Youll have 20g NaOH 20g NaOH80g water 100 20g NaOH every 100g of solution. NaOH Sodium hydroxide 50 1310-73-2. Liquid 480 480 480 480 480 480 480 480 480 480.
Sodium hydroxide 10 1310-73-2. Sodium hydroxide 40 1310-73-2. Sodium hydroxide 50 1310-73-2.
Liquid 480 480 480 480 480 480 480 480 480 480 indicates greater than. A blank cell indicates the fabric has not been tested. The fabric may or may not offer.
Nomenclature and molecular weight. Nafion can be produced as both a powder resin and a copolymer. Modern production methods produce Cl 2 and NaOHKOH from the electrolysis of brine using a Nafion membrane between half-cells.
Before the use of Nafion industries used mercury containing sodium amalgam to separate sodium metal from cells or asbestos diaphragms to allow for transfer of. Molecular FormulaNaOH Molecular Weight40 Section 10 - Stability and Reactivity Chemical Stability. Stable at room temperature in closed containers under normal storage and handling conditions.
Moisture contact with water exposure to moist air or water prolonged exposure to air. NBS Molecular Training Class April 25 2016. Stanimila Nikolova PhD.
Molecular Quality Improvement Program. Lets Talk About Solutions Solution - a homogeneous mixture of two or more substances. Solute - a substance in a solution that is present in the smallest amount.
Solvent - a substance in a solution that is present in the largest amount. Solubility - ability of the solute to dissolve in. It is also considered as a measure of the average molecular weight or chain length of all the fatty acids present.
The long chain fatty acids found in fats have low saponification value because they have a relatively fewer number of carboxylic functional groups per unit mass of the fat and therefore high molecular weight. Fats triglycerides upon alkaline hydrolysis either with. Cilostazol sold under the brand name Pletal among others is a medication used to help the symptoms of intermittent claudication in peripheral vascular disease.
If no improvement is seen after 3 months stopping the medication is reasonable. It may also be used to prevent stroke. It is taken by mouth.
The molecular weight of the nicotine is 1622g per mole. A certain sugar has a chemical composition of 40 carbon 66 hydrogen and 533 percent oxygen. The molar mass is 180 gmol.
A liquid which is soluble in water may be either a low molecular weight polar compound of up to 5 carbon atoms or less. You may add additional water up to 1 mL if your compound does not completely dissolve with the smaller amount. Check the pH of the water to determine if your unknown is partially or completely soluble in water and whether your compound has changed the pH of the water.
Sodium hydroxide NaOH weighs 2 130 kgm³ 13297156 lbft³ weight to volume volume to weight price mole to volume and weight mass and molar concentration density Volume to weight weight to volume and cost conversions for Refrigerant R-500 liquid R500 with temperature in the range of -5112C -60016F to 6834C 155. If these popular molecular weight MW DNA standards or other RNA standards are not available. For each 100 μl DNA in TE buffer add 10 μl 2 N NaOH and incubate at 37C for 10 min.
Add 40 μl 20 SSC and then place the sample on ice it will not be applied to the membrane immediately. As an alternative to the addition of 20 SSC one may add an equal volume of 2 M NH 4 OAc pH 70 and. Human abundance by weight.
This involves treatment with sodium hydroxide NaOH solution which results in a solution of sodium aluminate and sodium silicate. The iron remains behind as a solid. When CO 2 is blown through the resulting solution the sodium silicate stays in solution while the aluminium is precipitated out as aluminium hydroxide.
The hydroxide can be filtered off washed. The molecular weight 12114 gmol of Tris. Moles needed gmol g 3 Dissolve Tris Base in Water.
Dissolve the required mass of Tris into a volume of deionized water approximately 13 of the desired volume of buffer to be made. 4 Adjust the pH Using a pH meter titrate the solution of Tris with 1M hydrochloric acid HCl until the correct pH is reached. 5 Bring to Volume Add the TrisHCl.