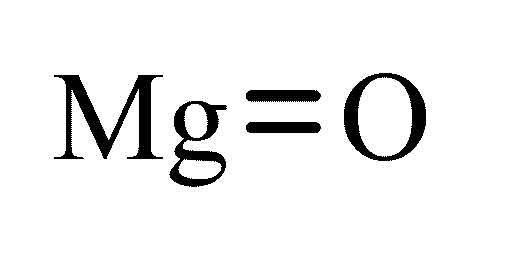
MgCl 2 is a molecular formula for magnesium chloride. Molecular mass of MgOM Atomic mass of Mg x 1 Atomic mass of O x 1 24 x 1 16 x 1 24 16 Molecular mass of MgOM 40 According to the formula Number of moles in the given MgO n.
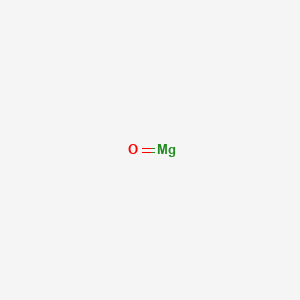
Of magnesium oxide can be calculated using the following experiment which finds the mass.
Molecular weigh magnesium oxide. To experimentally determine the empirical formula of magnesium oxide based on reaction stoichiometry. Introduction The molecular formula usually shortened to simply formula of a compound gives the number of atoms in each molecule of that compound. For example the formula for glucose C 6H 12O 6indicates that there are 6 atoms of C 12 atoms of H and 6 atoms of O in one molecule of.
Magnesium oxide for example the most common form. The amount of elemental magnesium in a supplement is the number of milligrams the magnesium atoms weigh in a magnesium compound. It is the actual weight of the magnesium atoms themselves.
Remember supplemental magnesium is never 100 magnesium because that would be a metal. Magnesium supplements are always together in. Number of moles of Magnesium oxide MgOn 02 mol To find.
Mass in grams of 02 mol of MgO Solution. Molecular mass of MgOM Atomic mass of Mg x 1 Atomic mass of O x 1 24 x 1 16 x 1 24 16 Molecular mass of MgOM 40 According to the formula Number of moles in the given MgO n. Of magnesium oxide can be calculated using the following experiment which finds the mass.
Of the magnesium and oxygen atoms. In a sample of the compound. Weigh a crucible.
Students weigh magnesium and heat it in a crucible. The magnesium reacts with oxygen to produce the oxide. Students see there is an increase in mass and can use the results to find the formula of magnesium oxide.
Video support and linked resources. This experiment is included in our Conservation of mass video along with supporting resources including illustrated technician notes integrated. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. Oxide is then reacted with acid to form copper II sulfate CuSO 4.
Finally the copper ions in the copper sulfate are reduced to copper metal by magnesium. In theory you should be able to recover all of the copper that you started with. The number of atoms of copper is the same throughout all of the reactions However small amounts of copper can be lost during transfers of copper.
Atomic Radius of Magnesium. The atomic radius of Magnesium atom is 141pm covalent radius. It must be noted atoms lack a well-defined outer boundary.
The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However this assumes the atom to exhibit a spherical shape which. For compounds the molecular mass in amu is numerically the same as the mass of one mole of the compound in grams.
Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a. Calculate the number of moles of magnesium oxide MgO in a 80 g and b 10 g of the compound.
As per the periodic table relative atomic mass of Oxygen O 16 and Magnesium Mg 24. Mass of 1 Mole of MgO. 1 x 24 1 x 16 40 g a Number of Moles frac MassMolar Mass frac 8040 2 Moles.
Heavy water deuterium oxide 2 H 2 O D 2 O is a form of water that contains only deuterium 2 H or D also known as heavy hydrogen rather than the common hydrogen-1 isotope 1 H or H also called protium that makes up most of the hydrogen in normal water. The presence of the heavier hydrogen isotope gives the water different nuclear properties and the increase of mass gives it slightly. The compound recipe contains 100 phr E-60C precompound 30 phr MT black 6 phr calcium hydroxide and 3 phr magnesium oxide.
The clean mill is cooled to about 25C and the nip is adjusted to about 3 mm. The polymer is added to the mill for banding. Ordinarily the fluoroelastomer bands on the fast roll but may be forced to the slow roll by increasing the temperature slightly on the slow roll.
This property enables the graphite oxide to be exfoliated in water using sonication ultimately producing single or few layer graphene known as graphene oxide GO. The main difference between graphite oxide and graphene oxide is thus the number of layers. While graphite oxide is a multilayer system in a graphene oxide dispersion a few layers flakes and monolayer flakes can be found.
For example 24Mg is pronounced magnesium 24 and can be written as Mg-24 or magnesium-24. 25Mg is pronounced magnesium 25 and can be written as Mg-25 or magnesium-25. All magnesium atoms in the nucleus have 12 protons.
The only difference between them is that the magnesium-24 atom in the nucleus has 12 neutrons the magnesium-25 atom has 13 neutrons and. MgCl 2 is a molecular formula for magnesium chloride. _ True o False.
Give the empirical formula for the compound below. C 2 H 4 _ C 2 H 4 _ CH o CH 2 _ C 2 H 2. Give the empirical formula for the compound below.
C 2 H 6 O 2 _ CHO _ C 2 H 2 O 2 o CH 3 O _ C 2 H 6 O 2. Give the empirical formula for the compound. Magnesium metal reacts with oxygen to make a white powder known as magnesium oxide.
As the reaction proceeds a bright white flame is seen. Magnesium nanoparticles of various mean diameters 53239 nm were synthesised in this study via pulsed laser ablation in liquid PLAL from millimetre sized magnesium powders within isopropyl alcohol. It was observed via a 3.
3 full factorial design of experiments that the processing parameters can control the nanoparticle distribution to produce three size-distribution. Graphene oxide is usually produced through chemical exfoliation of graphite. A particularly popular technique is the improved Hummers method.
204 Using paper-making techniques on dispersed oxidized and chemically processed graphite in water the monolayer flakes form a. Magnesium reacts slowly with water to generate magnesium oxide a weak soluble base and hydrogen gas. The reaction with heated magnesium is faster with steam and a white powder magnesium oxide is generated together with hydrogen.
If magnesium has been previously ignited in air it will burn with a dazzling white flame in steam. For patients who weigh 99 lbs or more Impavido is administered at a dose of three 3 of the 50-mg capsules per day for 28 days. Patients who weigh close to 99 lbs will receive more drug per pound than patients who have higher weights.
For example a patient who weighs 120 pounds will receive 125 mg of Impavido per pound each day 150 mg each. If one mole of carbon atoms weigh 12 grams what is the mass in grams of 1 atom of carbon. Magnesium chloride b Calcium oxide c Copper nitrate d Aluminium chloride e Calcium carbonate.
A Magnesium chloride Symbol Mg Cl Change 2 -1 Formula MgCl 2 b Calcium oxide Symbol Ca O Charge 2 -2 Formula CaO c Copper nitrate Symbol. Weigh out five separate 1 g portions of baking soda measure the temperature of the vinegar add 1 g of baking soda to the vinegar and stir record the new temperature add the other portions of baking soda stirring and recording the temperature after each portion is added The graph shows her results. 20 18 16 14 12 10.
MercuryII oxide an orange crystalline solid can be broken down by heat into the elements mercury and oxygen. When heated in the absence of air the compound sucrose is broken down into the element carbon and the compound water. The initial stage of this process when the sugar is turning brown is known as caramelizationthis is what imparts the characteristic sweet and nutty flavor.
You can view more details on each measurement unit. Molecular weight of HNO3 or grams This compound is also known as Nitric Acid. 45 mol BaSO4 B 1.
One mole of glucose C6H12O6 has a mass equal to 180. 1 u is equal to 112 the mass of one atom For example nitrogen monoxide NOg can be prepared in the lab by the following chemical reaction. And the magnesium ions in an Epsom salt bath are way smaller than 500 Daltons at a barely-there molecular weight of just 24 Daltons.
Im sure we can just stop there. Theres probably no debate or uncertainty about this probably no other obscure and technical chemistry considerations. The magnesium is small enough to get through case closed.