The electrical dipole moment of gaseous sulfur trioxide is zero. Units in gas specific gravity and ideal gas calculators.

In chemistry the empirical formula of a compound is the simplest positive integer ratio of the present atoms in the compound.
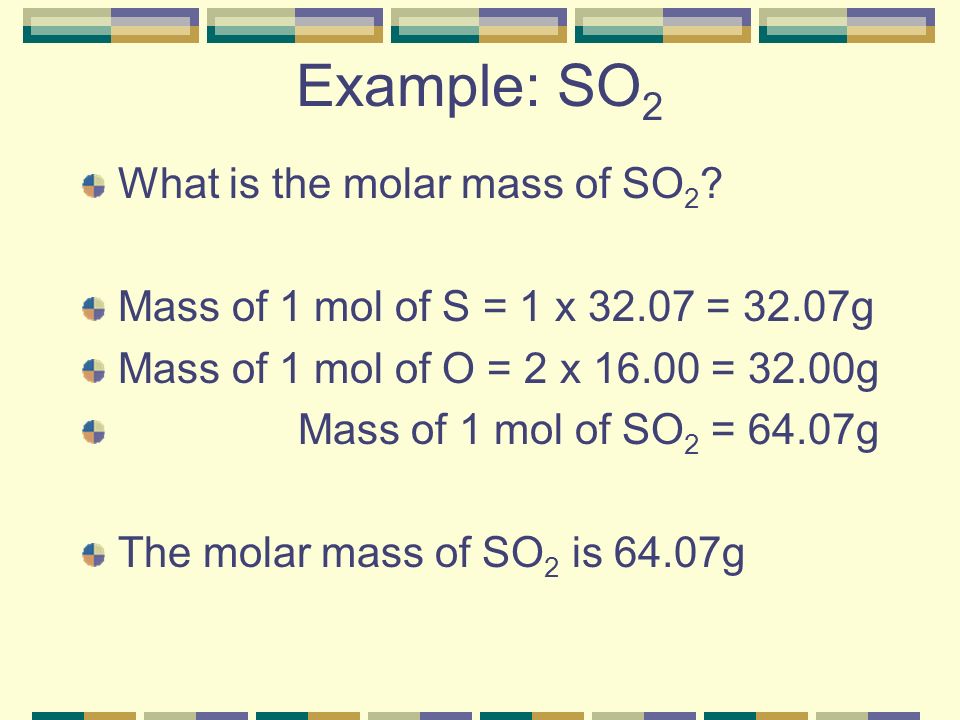
Molecular mass of sulfur dioxide. Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. Clarification neededOn other planets sulfur dioxide can be found in various concentrations the most significant being the atmosphere of Venus where it is the third-most abundant atmospheric gas at 150 ppmThere it reacts with water to form clouds of sulfuric acid and is a key. Molecular weight calculator is an online tool to calculate atomic mass and molecular mass.
Molecular mass calculator helps a user to complete his work in a given time without any errors. How many elements are in the periodic table. In a periodic table there are a total of 118 elements.
The tabular chart we see on the periodic table is arranged. Sulfur dioxide is highly soluble in aqueous media. Absorption after inhalation has been studied in rabbits and man.
In rabbits about 40 of the inhaled sulfur dioxide is absorbed in the nose and pharynx when concentrations of about 290 ugcu m 01 ppm are inhaled. At higher concentrations 29-290 mgcu m 10-100 ppm the fraction absorbed. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. Also important in this field is Avogadros number N.
What is relative formula mass and relative molecular mass. How many grams of sulfur are in the sample. Show Video Lesson.
How to calculate the Mass Percent of an Element in a Compound. Mass Percent Composition of an Element in a Compound To calculate the mass percent composition or simply the mass percent of an element in a compound we divide the mass of the element in 1 mol of. PCAM 204 8 uses a solid sorbent molecular sieve 5A thermal desorption and mass spectrometry.
NIOSH Manual of Analytical Methods NMAM Fourth Edition 81594. METHOD 6004 Issue 2 dated 15 August 1994 - Page 2 of 4 REAGENTS. Water deionized filtered specific conductance 10 µScm.
Dissolve 25 g Na 2CO 3 in deionized water. Add 20 mL. This allows the chemist to weigh two substances such as sulfur and iron to obtain the same number of sulfur and iron atoms.
What is the empirical formula. In chemistry the empirical formula of a compound is the simplest positive integer ratio of the present atoms in the compound. Why we use Monoisotopic mass Mmi.
Mmi is one of the different types of molecular weights used in mass. Calculate the empirical and molecular formula of ethylene glycol given its molar mass 60 gmol 60 amumolecule When given the by mass values of each atom in a compound assume a 100 g sample - the and g values are then the same. Molar mass is useful in converting between units of grams and molesAtomic mass unit or amu.
The atomic mass unit amu is equal to th. Molecular structure and bonding. The molecule SO 3 is trigonal planarAs predicted by VSEPR theory its structure belongs to the D 3h point groupThe sulfur atom has an oxidation state of 6 and a formal charge of 0.
The S-O bonding is delocalized with all three S-O bond lengths equal at 142 Å. The electrical dipole moment of gaseous sulfur trioxide is zero. Sulfurous acid is also called Sulfur dioxide solution or dihydrogen trioxosulfate or trioxosulfuric acid.
Molecular weight of H 2 SO 3. Of hydrogen bond acceptor. Monoisotopic mass of Sulfurous Acid.
Of hydrogen bond donor. Sulfurous Acid Structure H 2 SO 3. Sulfurous Acid Structure H2SO3.
Sulfurous Acid Uses H 2 SO 3 Sulfurous Acid is used. In juices with high acidity less sulfur dioxide is required for example 15 mg l1 with free sulfur dioxide at pH 30 has the same antimicrobial effect as 150 mg l1 at pH 40 Percentage of sulfite bisulfite and molecular sulfur dioxide in aqueous solution as a function of the pH. Sulfur dioxide SO 2 in the.
We have used air mass factors from the operational product. Note that doing so is not strictly valid because one should expect lower AMFs due to the change in fitting window from 3105 to 326 to 312326 nm. To account for this we have applied a constant scaling factor of 115 to the retrieved SO 2 VCDs.
Based on radiative transfer calculations we found. 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 L of a gas at STP You do not need to worry about this yet Each definition can be written as a set of two conversion factors. 1 mole molar massg can be written as ____1 mole OR _molar mass g.
A sample of sulfur having a mass of 128 g combines with oxygen to form a compound with a mass 320 g. What is the empirical formula of the compound. What is the empirical formula.
The analysis of a hydrocarbon revealed that it was 856281 C and 143719 H by mass. When 341 g of the gas was stored in a 14 L flask at -866273 C it exerted a pressure of 505 Torr. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. To calculate the molecular weight of ethanol the molecular weight of each atom in the molecule is summed. M ethanol 212011kgkmol 61008kgkmol 115999 kgkmol 46069 kgkmol See also Physical data for hydrocarbons Physical data for alcohols and carboxylic acids Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds.
The 13X molecular sieve is the sodium form of zeolite X and has a much larger pore opening than the type A crystals with an effective pore diameter of 10 angstroms. It also has the highest theoretical capacity of the common adsorbents and excellent mass transfer rates. Type 13X offers enhanced adsorption performance over the type A zeolite.
Since we know the molar mass of each substance we can also establish the mass relationships. CH 4 2O 2 CO 2 2H 2O 1 mole 2 moles 1 mole 2 moles x 160 gmol 320 gmol 440 gmol 180 gmol 160 g 640 g 440 g 360 g The total mass of products should be the same as the total mass of reactants. The molar mass is simply the mass of one mole of substance ie.
The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. The unit of molar mass in the SI system is kilogram per mole. Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component.
Benzene Gas - Specific Heat - Specific heat of Benzene Gas - C6H6 - at temperatures ranging 250 - 900 K. Biogas - Carbon Nitrogen Ratios - Carbon - Nitrogen ratios for biogas produced from various raw materials. Since a molecule of carbon dioxide is composed of only three atoms its mass spectrum is very simple.
The molecular ion is also the base peak and the only fragment ions are CO mz28 and O mz16. The molecular ion of propane also has mz44 but it is not the most abundant ion in the spectrum. Cleavage of a carbon-carbon bond gives methyl and ethyl fragments one of which is a.
Units in gas specific gravity and ideal gas calculators. Atmatmosphere CCelsius cmcentimeter FFahrenheit ftfoot ggram kgkilogram mmeter mmmillimeter NNewton PaPascal psipound per square inch. Equations for Gas Specific Gravity and Molecular Weight Conversion.
S M M air where Sgas specific gravity Mgas molecular weight M air 2896443 gmole molecular weight of. Particulate matter that consists of compounds of sulfur formed by the interaction of sulfur dioxide and sulfur trioxide with other compounds in the atmosphere. Sulfate aerosols are injected into the atmosphere from the combustion of fossil fuels and the eruption of volcanoes like Mt.
Sulfate aerosols can lower the Earths temperature by reflecting away solar radiation negative. SO 2 - sulfur dioxide SF 6 - sulfur hexafluoride CCl 4 - carbon tetrachloride NI 3 - nitrogen triiodide. Writing the Formula From the Name.
You can write the formula for a covalent compound from its name by writing the symbols for the first and second elements and translating the prefixes into subscripts. For example xenon hexafluoride would be written XF 6. It is common for students to have.