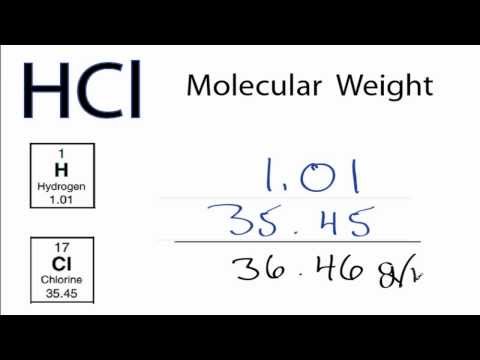
What is the mass of 0288 moles of plumbous. Molar mass is an important concept in adapting chemical formulas to real world conditions.

For example if the formula says 2H 2 O in the chemical equation DONT use 360 gmol use 180 gmol.
Molar mass of hydrogen chloride. For hydrogen chloride HCl the molar mass of each element is 1007 grams per mole for hydrogen and 35453 grams per mole for chlorine. For glucose C 6 H 12 O 6 the molar mass of each element is. Carbon 120107 x 6 720642 gmol.
Hydrogen 1007 x 12 12084 gmol. And oxygen 159994 x 6 959964 gmol. Add the molar masses of each element in the compound.
Hydrogen molecule H2 - Molecular mass molar mass. Type the number of Hydrogen molecule H2 you want to convert in the text box to see the results in the table. Gram per mole gmol-Kilogram per mole kgmol- Standard atomic weight.
Hydrogen H-Oxygen O-Sulfur S-Chlorine Cl-Iron Fe- Molecular mass. Hydrogen molecule H2-Water molecule. The molar mass is simply the mass of one mole of substance ie.
The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. The unit of molar mass in the SI system is kilogram per mole. The molar mass links the mass of a substance to its moles.
Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. Let me make it more clear with an example of sodium chloride. The molar mass of sodium chloride is known.
It is 5844 g mol 1. If we have to measure one mole of sodium. The relative atomic mass of a compound is the ratio of the average mass of the elements in a chemical compound to the atomic mass constant which is defined as 112 the mass of a carbon 12 atom.
For a single sample the relative atomic mass of the sample is the weighted arithmetic mean of the masses of the individual atoms present in the sample also known as the average atomic mass. Measurement units Molar Mass Conversion Molar Mass Converter Chlorine Cl - standard atomic weight Chlorine molecules Cl₂ - molecular mass Grams per molegmol Hydrogens H - standard atomic weight Hydrogen molecules H₂ - molecular mass Iron Fe - standard atomic weight Kilograms per molekgmol Oxygen O - standard atomic weight Sulfur S - standard atomic weight. 136315 gmol Appearance white crystalline solid hygroscopic and very deliquescent Odor.
290 C 554 F. 563 K Boiling point. 732 C 1350 F.
1005 K Solubility in water. 4320 g 100 g 25 C Solubility. Soluble in ethanol glycerol and acetone.
4300 g100ml Magnetic susceptibility χ 65010. Molar Mass of Frequently Calculated Chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
Molar mass is an important concept in adapting chemical formulas to real world conditions. We may be able to balance a chemical equation and determine that one molecule of hydrogen combines with two of oxygen to make water or the compound of your choice. But how would you set up the materials in the laboratory.
Or if you were for example buying oxygen for a process how would you determine. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
Hydrogen chloride is a diatomic molecule consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bondThe chlorine atom is much more electronegative than the hydrogen atom which makes this bond polar. Consequently the molecule has a large dipole moment with a negative partial charge δ at the chlorine atom and a positive partial charge δ at the hydrogen. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance.
The representative particles can be atoms molecules or formula units of ionic compounds. This relationship is frequently used in the laboratory. The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion or its reverse a mass-mole.
Therefore the mass of a sodium-23 atom is 3817 10 23 g. To Determine Molecular Mass of Iodine gas. The atomic mass of iodine is 1269 g mol 1.
Determine the molecular mass of iodine gas. The iodine gas is a diatomic gas. The molecular formula is I 2.
So the molar mass of I 2 is twice the molar mass of I. Number of Moles frac MassMolar Mass Molar Mass frac MassNumber of Moles Molar Mass frac 10001574 Molar Mass 5844 gmol This mole conversion calculator also helps you calculate molar mass of a substance using a similar mathematical approach but in less time. If the substance is an element then the.
Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.
What is the molar mass of. Make sure to show clearly defined and complete work here 1. Conversion between moles and mass.
How many moles are in 1225 g of magnesium hydroxide. How many moles are in 245 g of hydrogen gas H2. How many grams are in 63 moles of ammonium chloride.
What is the mass of 0288 moles of plumbous. As with all these problems it is usually assumed that we have a 100g mass of unknown compound and we work out the molar quantities. And thus moles of carbon -80g12011gmol-1666mol.
And thus moles of hydrogen -20g1008gmol-1198mol. We divide the molar quantities thru by the SMALLER molar quantity. The molar mass is the mass in grams of 1 mole of a particular molecule.
How to find molar mass. One mole of sodium Na is 2299 g and 1 mole of chlorine is 3545 g. For sodium chloride NaCl they are in a ratio of 11 so the molar mass of NaCl is 2299 3545 5844 gmol.
For a compound like water H 2 O 1 mole of hydrogen H is 1008 gmol and 1 mole of oxygen O is 159994 gmol. Bioaccumulation Estimates from Log Kow BCFWIN v217. Log BCF from regression-based method 0500 BCF 3162 log Kow used.
054 estimated Volatilization from Water. 466E-014 atm-m3mole calculated from VPWS Half-Life from Model River. 7591E009 hours 3163E008 days Half-Life from Model Lake.
8281E010 hours 345E009 days Removal In Wastewater Treatment. PV nRT n mass g molar mass gmol PV mass RT mass x R x T molar mass molar mass P x V Knowing that the units for density are massvolume re-write this equation so that it equates density with molar mass. Molar mass P dRT V mass d 6.
Hydrochloric acid solution is a colorless watery liquid with a sharp irritating odor. Consists of hydrogen chloride a gas dissolved in water. Sinks and mixes with water.
USCG 1999 CAMEO Chemicals. At room temperature hydrogen chloride is a colorless to slightly yellow corrosive nonflammable gas that is heavier than air and has a strong irritating odor. How many moles are in 82 grams of hydrogen chloride HCl.
The atomic mass of H is 1007 and Cl is 35453 making the molar mass of the compound 1007 35453 3646 gmol. Dividing the number of grams of the substance by the molar mass yields. 82 g 3646 gmol 0225 moles of HCl.
Dont multiply the molar mass of a substance by the coefficient in the problem BEFORE using it in one of the steps above. For example if the formula says 2H 2 O in the chemical equation DONT use 360 gmol use 180 gmol. Dont round off until the very last answer.
In other words dont clear your calculator after step two and write down a value of 3 or 4 significant figures to use in the. 182 Occurrence and Preparation of the Representative Metals. 183 Structure and General Properties of the Metalloids.
184 Structure and General Properties of the Nonmetals. 185 Occurrence Preparation and Compounds of Hydrogen. 186 Occurrence Preparation and Properties of Carbonates.
187 Occurrence Preparation and Properties of Nitrogen. Strong Diprotic Acid and Strong Monobasic Base. The experiment described above is repeated using 500 mL of 10 mol L-1 sodium hydroxide a strong monobasic base and 10 mol L-1 sulfuric acid a strong diprotic acid instead of 10 mol L-1 hydrochloric acid a strong monoprotic acid.
When plotted on a graph as shown below the second experiments results look.