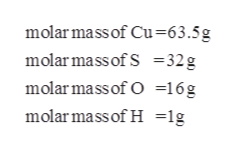
The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water. 0001 mg Cd 2 in 0100 L the maximum permissible concentration of cadmium in drinking water.

How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution.
Molar mass copper 2 sulfate. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Four types of crystal size are provided based on its usage. Large crystals 1040 mm small crystals 210 mm snow crystals less than 2 mm and windswept powder less than 015 mm.
Molar Mass of Frequently Calculated Chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Copper Sulfate and water formed. Copper Sulfate is a blue precipitate but since it is soluble in water it dissolves and the mixture is turned a bright blue color.
Zinc sulfate is formed and is aqueous. Hydrogen gas is also formed and released. Solid copper is found on the bottom of the beaker.
Net Ionic Equations 1. Cu s HNO 3 aq NIE. Cus 4H aq 2NO 3-aq— Cu 2 aq 2NO.
Copper hydroxide -CuOH2 - Copper hydroxide is a crystalline inert compound used in the preparation of a wide variety of salts. Copper hydroxide is also called cupric hydroxide. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water.
To learn more about the Structure Physical Properties Chemical Properties Uses and FAQs of Copper hydroxide Visit BYJUS for more content. Calculate the molar masses of H 2 O and CuSO 4 Relative atomic masses. H1 O16 S32 Cu64 Calculate the mass of water driven off and the mass of anhydrous copperII sulfate formed in your experiment.
Calculate the number of moles of anhydrous copperII sulfate formed. Calculate the number of moles of water driven off. Calculate how many moles of water would have been driven off if 1.
The molar mass of the anhydrous and the pentahydrate forms of copper sulfate are 159609 gramsmole and 249685 grams per mole respectively. Anhydrous CuSO 4 has a grey-white powdery appearance whereas the pentahydrate has a bright blue colour. For instance the copper sulfate used earlier in the semester was stated to be CuSO 4 but it actually had absorbed 5 moles of water for every 1 mole of CuSO 4 and should have been correctly labeled as CuSO 45H 2O copper II sulfate pentahydrate.
The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. The loss in mass of the compound after. In this experiment the hydrates of copperII sulfate CuSO.
O and magnesium sulfate MgSO. O will be studied. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water.
The mass of water lost during heating can be determined and the coefficient. Can be calculated from the ratio of moles. In the case of copperII sulfate hydrates you only get x as a whole number.
Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. For example copperII pentahydrate CuSO_4 5H_2O is blue. On the other hand.
The law that the scientist used to predict that the product of the reaction would be 159 g of copper sulfate is the law of conservation of matter mass The la Sky0607 Sky0607 10122021 Chemistry High School answered expert verified 50 POINTS. Copper Cu reacts with sulfur S to form copper sulfide as shown in the equation. A scientist adds 127 grams of Cu to 32 grams of S to.
What is the mass of 356 x 1025 formula units of copper II sulfate. What is the number of molecules present in 5000 g of diphosphorus pentoxide. What is the mass of 125 x 1023 sodium ions.
Worksheet Mole Conversions Name. Show all work utilizing dimensional analysis wherever possible. Include all units and account for significant figures.
What is the molar mass of. Make sure to. Copper sulfate injected ip at 2 mg copperkg into vitamin e selenium deficient rats caused a 6 fold increase in the formation of the lipid peroxidation product ethane caused acute mortality in 45 rats.
Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. 1 mole molar mass could be atomic mass from periodic table or molecular mass 1.
How many moles are in 68 grams of copper II hydroxide CuOH 2. 13 How many grams are in 33 moles of potassium sulfide K 2 S. 14 How many moles are in 12 x 103 grams of ammonia NH 3.
15 How many grams are in 23 x 10-4 moles of calcium phosphate Ca 3 PO 3 2. 16 How many moles are in 34 x. Calculate the molar mass of the empirical formula 3.
Take the molar mass of the molecular formula given in the problem and divide it by the molar mass of the empirical formula. This will equal the number of times the ef. Is in the mf.
Distribute the number attained through the empirical formula Example. 2C 2 H 5 C 4 H 10 Calculating the Formula of a Hydrate 1. Determine the mass.
What is the percent water in copperII sulfate pentahydrate CuSO 4 5 H 2 O. Calculate the formula mass. When determining the formula mass for a hydrate the waters of hydration must be included.
1 Cu6355 gmol 1 S3207 gmol 4 O1600 gmol 15962 gmol Formula mass 15962 gmol 5 H 2 0 1802 g H 2 0mol 24972 gmol 2. Divide the mass of water in one mole. In terms of atomic masses.
In a total of 14202 parts by mass of sodium sulfate 4598 parts are sodium 3206 parts are sulfur and 6400 parts are oxygen. In terms of a gram mass unit molar mass. In a total of 14204 g of sodium sulfate 4598 g are sodium 3206 g are sulfur and 6400 g are oxygen.
CuNO326H2O hydrated copper II nitrateV. CuNO32 Anhydrous copper II nitrateV. XH2O has a molar mass of 3221 Calculate the value of x Molar mass xH2O 3221 23x2 321 16x4 180 X 18018 10.
Find the molar mass of copper on the periodic table. The mole often abbreviated as mol listed above is a unit of measurement. If you sold eggs you would talk about them in the dozens not one by one.
A mole is a certain amount too. If chemists want to speak about incredibly small atoms and molecules an amount far greater than a dozen is needed. Molar mass Mr for a compound can be calculated by adding up the mass numbers f rom the periodic table of each element in the compound eg CaCO3 401 120 160 x3 1001 1.
For pure solids liquids and gases Unit of mass. Grams Unit of moles. Mol Significant Figures Give your answers to the same number of significant figures as the number of significant figures for the data you given.
2026 g FeCl 3 in 01250 L of a solution used as an unknown in general chemistry laboratories. 0001 mg Cd 2 in 0100 L the maximum permissible concentration of cadmium in drinking water. 00079 g C 7 H 5 SNO 3 in one ounce 296 mL the concentration of saccharin in a diet soft drink.
There is about 10 g of calcium as Ca 2 in 10 L of milk. Aluminum sulfate Al 2 SO 4 3 is an ionic compound that is used in the manufacture of paper and in various water purification processes. What is the formula mass amu of this compound.
Solution The formula for this compound indicates it contains Al 3 and SO 4 2 ions combined in a 23 ratio. For purposes of computing a formula mass it is helpful to rewrite the formula in the simpler. A laboratory procedure calls for making 4533 ml of a 262M salt solution.
If the molar mass of the salt is 218 gmol what mass is required. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution.
What volume of 12 M HCl solution is. The net ionic equation for formation of a precipitate note correct spelling when sodium sulfate and barium chloride solutions are mixed is Ba2 SO4 -2 -.